Molecule dispersion type white light polymer material and its preparation method
A polymer material and molecular dispersion technology, which is applied in the field of molecular dispersion white light polymer materials and its preparation, can solve the problems that there is still a certain distance for practical application, and achieve the effect of spectral stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
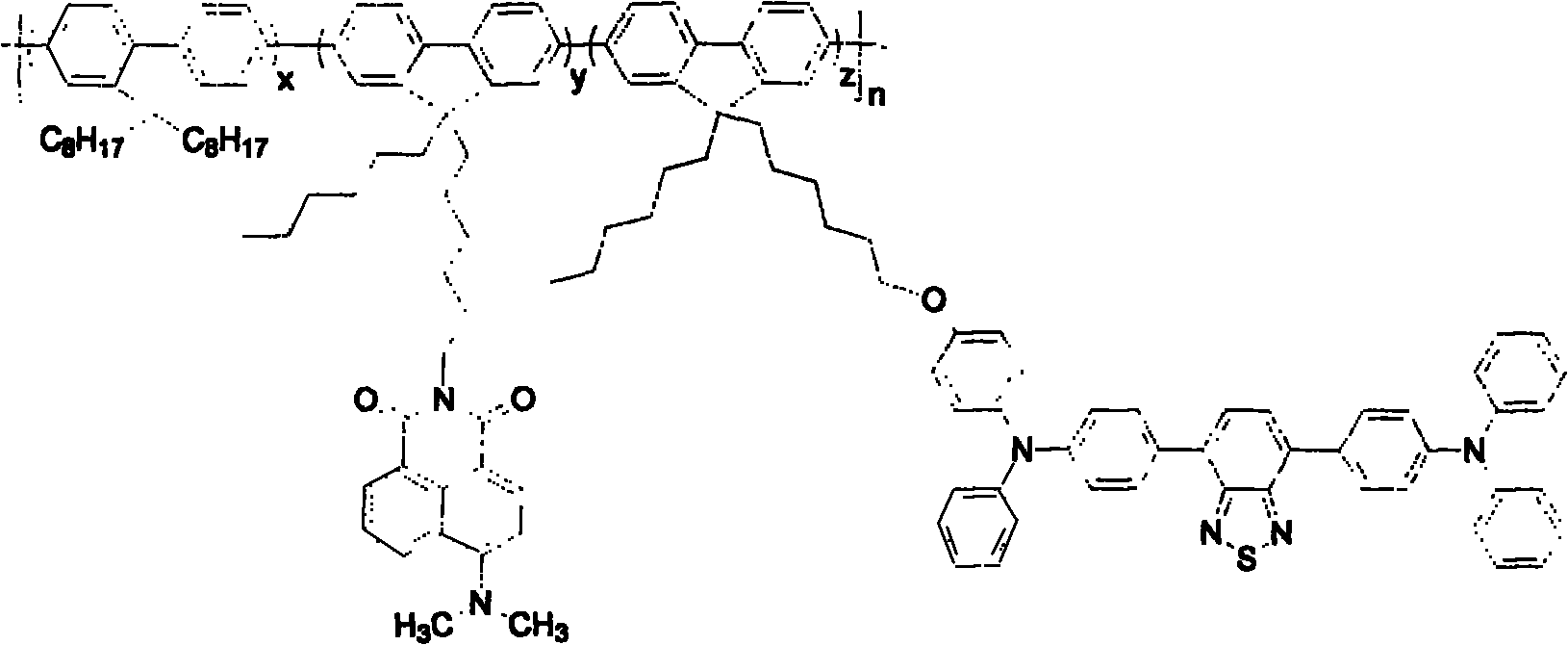
[0024] Example 1: Synthesis of 9-hexyl-9-(6-(4-amino-1,8-naphthalimide-4-)hexyl)-2,7-dibromofluorene
[0025] Under argon protection, add 2.12g (10mmol) 4-amino-1,8-naphthalimide and 60ml dimethyl sulfoxide to the reaction flask, heat to 120°C, add 0.56g (10mmol) ) Potassium hydroxide, after 10 minutes, add 5.72g (10mmol) 9-hexyl-9-(6-bromohexyl)-2,7-dibromofluorene to the reaction flask, and react for 10 hours. After the reaction, the reaction mixture was poured into water, extracted with dichloromethane, washed three times with water, dried over anhydrous sodium sulfate, concentrated, and the obtained crude product was separated by a silica gel column to obtain 4.97g 9-hexyl-9-(6-(4 -Amino-1,8-naphthoimide-4-)hexyl)-2,7-dibromofluorene, as a yellow solid, yield: 71%.
Embodiment 2
[0026]Example 2: Synthesis of 9-hexyl-9-(6-(4-dimethylamino-1,8-naphthalimide-4-)hexyl)-2,7-dibromofluorene
[0027] Under argon protection, add 1.41g (2.0mmol) 9-hexyl-9-(6-(4-amino-1,8-naphthalimide-4-)hexyl)-2,7- Dibromofluorene and 20ml dimethyl sulfoxide, add 0.24g (10mmol) 50% sodium hydride to the reaction flask at room temperature, after 10 minutes, add 1.52g (10mmol) methyl iodide to the reaction flask, and react for 5 hours. After the reaction, the reaction mixture was poured into water, extracted with dichloromethane, washed three times with water, dried over anhydrous sodium sulfate, concentrated, and the obtained crude product was separated by a silica gel column to obtain 1.33g 9-hexyl-9-(6-(4 -Dimethylamino-1,8-naphthoimide-4-)hexyl)-2,7-dibromofluorene, yellow solid, yield: 91%.
Embodiment 3
[0028] Example 3: Synthesis of 4-bromo-7-(4-(diphenylamino)phenyl)-2,1,3-benzothiadiazole
[0029] Under argon protection, 2.94g (10mmol) 4,7-dibromo-2,1,3-benzothiadiazole, 2.67g (5mmol) tributyl (4-(diphenyl Amino)phenyl)tin, 0.056g (0.05mmol) tetrakis(triphenylphosphine)palladium, 100ml toluene, react at 100°C for 24 hours. Then cooled to room temperature, the reaction mixture was washed three times with water, dried over anhydrous sodium sulfate, concentrated, and the obtained crude product was separated by silica gel column to obtain 1.74g 4-bromo-7-(4-(diphenylamino)phenyl)- 2,1,3-Benzothiadiazole, orange solid, yield: 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com