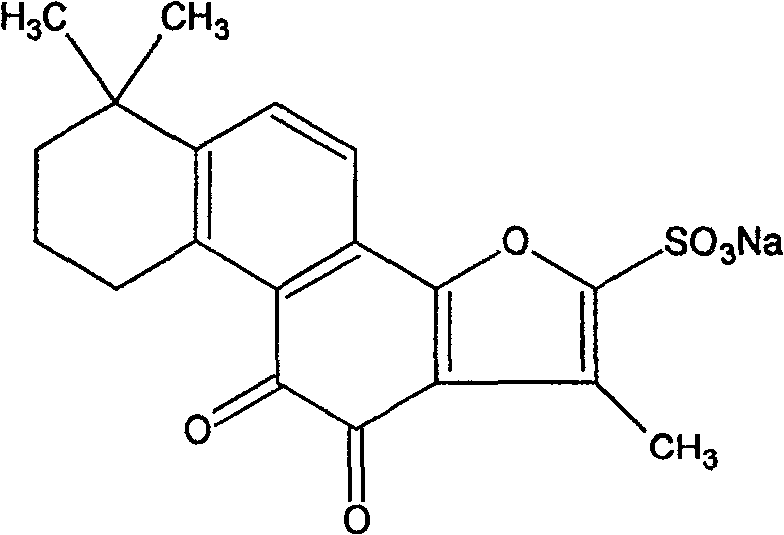
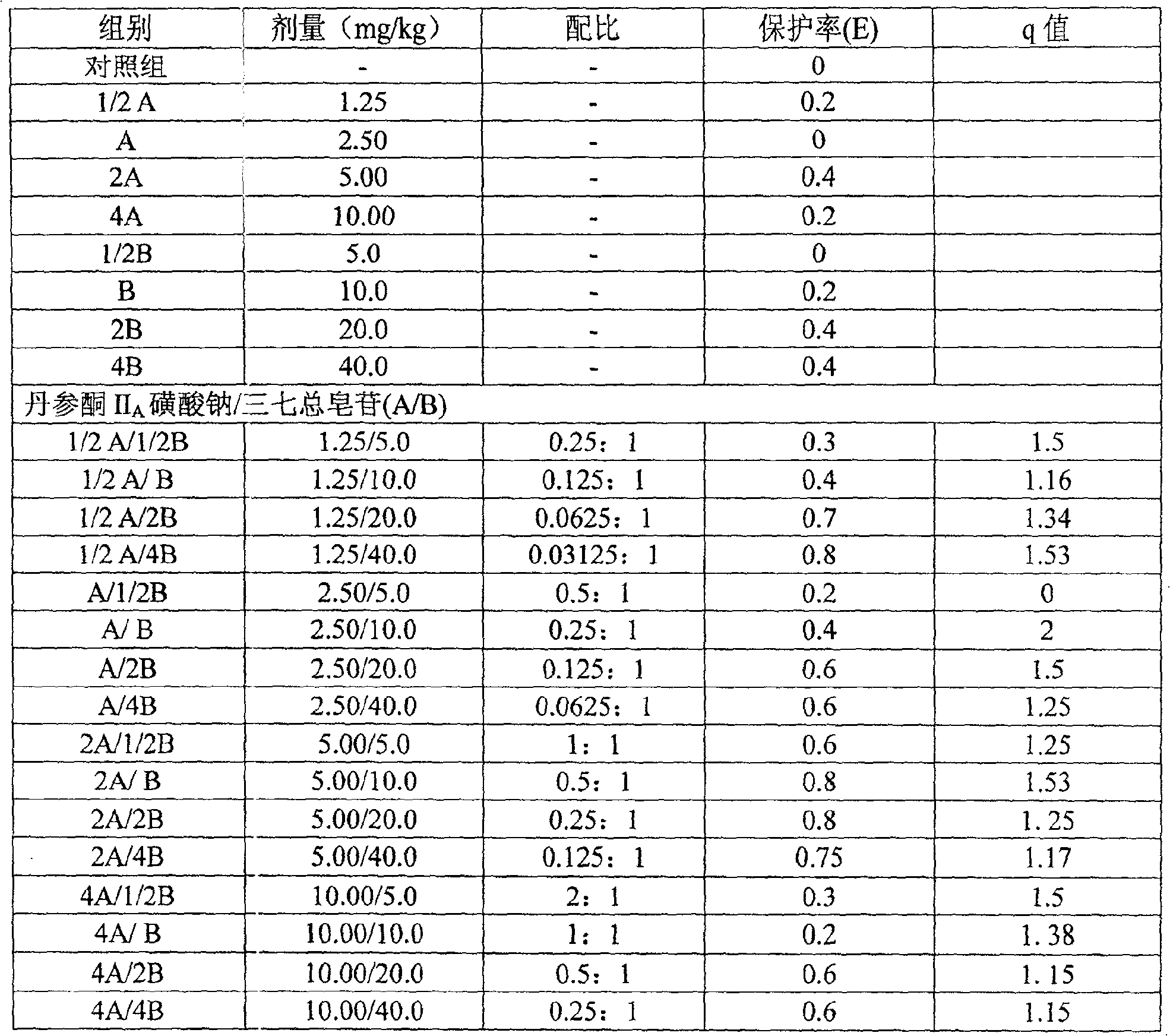
Composition of notoginsen triterpenes and tanshinone IIA sodium sulfonate
A technology of Panax notoginseng saponins and a composition, which is applied in the field of medicine and achieves the effects of improved drug efficacy, less impurities, and uniform and stable drug quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Example 1: Panax notoginseng saponins and tanshinone II A Preparation of sodium sulfonate water injection
[0112] prescription:
[0113] Panax notoginseng saponins
120g
Sodium Tanshinone IIA Sulfonate
12g
Propylene Glycol
800ml
Water for Injection
Add to 1000ml
co-preparation
1000 pieces
[0114] Panax notoginseng saponins
120g
Tanshinone II A Sodium sulfonate
30g
Propylene Glycol
800ml
Water for Injection
Add to 1000ml
co-preparation
1000 pieces
[0115] Panax notoginseng saponins
120g
Tanshinone II A Sodium sulfonate
240g
Propylene Glycol
800ml
Water for Injection
Add to 1000ml
co-preparation
1000 pieces
[0116] Preparation Process:
[0117] 1) Dispose of the pipes and containers used for liquid preparation one day in advance, and rinse them wi...
Embodiment 2
[0128] Example 2: Panax notoginseng saponins and tanshinone II A Preparation of sodium sulfonate powder injection
[0129] prescription:
[0130] Panax notoginseng saponins
120g
Tanshinone II A Sodium sulfonate
12g
Polysorbate 80
100g
Mannitol
300g
sterile water for injection
Add to 3000ml
co-preparation
1000 pieces
[0131] Panax notoginseng saponins
120g
Tanshinone II A Sodium sulfonate
30g
Polysorbate 80
125g
Mannitol
350g
sterile water for injection
Add to 3500ml
co-preparation
1000 pieces
[0132] Panax notoginseng saponins
120g
Tanshinone II A Sodium sulfonate
240g
Polysorbate 80
200g
Mannitol
450g
sterile water for injection
Add to 4000ml
co-preparation
1000 pieces
[0133] Preparation Process:
[0134] 1) Firs...
Embodiment 3
[0144] Example 3: Panax notoginseng saponins and tanshinone II A Preparation of sodium sulfonate infusion
[0145] prescription:
[0146] Sodium chloride infusion:
[0147] Panax notoginseng saponins
120g
Tanshinone II A Sodium sulfonate
12g
Polysorbate 80
100g
Sodium chloride
900g
Water for Injection
Add to 100000ml
co-preparation
1000 bottles
[0148] Panax notoginseng saponins
120g
Tanshinone II A Sodium sulfonate
30g
Polysorbate 80
125g
Sodium chloride
900g
Water for Injection
Add to 100000ml
co-preparation
1000 bottles
[0149] Panax notoginseng saponins
120g
Tanshinone II A Sodium sulfonate
240g
Polysorbate 80
200g
Sodium chloride
900g
Water for Injection
Add to 100000ml
co-preparation
1000 bottles
[0150...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com