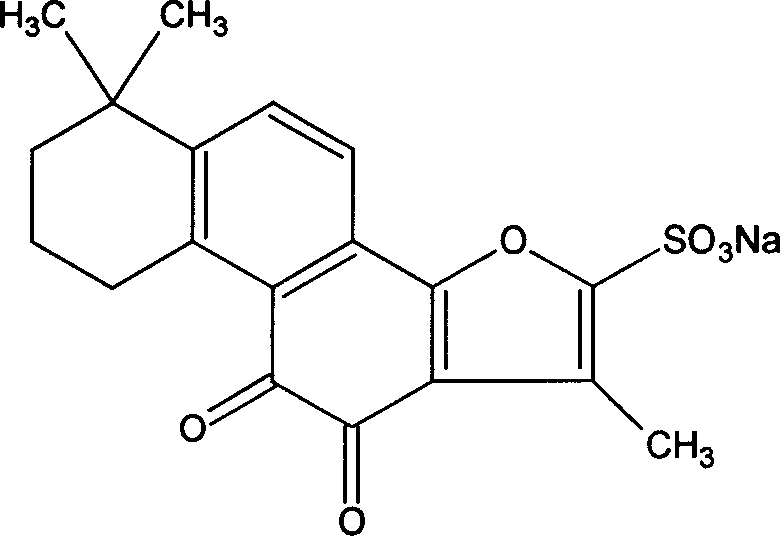
Composition of notoginsen triterpenes and tanshinone IIA sodium sulfonate
A technology of Panax notoginseng saponins and composition, which is applied in the field of medicine to achieve the effects of high drug purity, avoiding drug loss, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0018] Experimental example 1: Panax notoginseng saponins and tanshinone II A Study on the efficacy of sodium sulfonate in combination with medication
[0019] Test animals: mice, male, weighing 22±8g, 250 in number, 10 in each group.
[0020] The mice were observed for one week in a breeding and observation room, the temperature of the observation room was 20±3°C, the relative humidity was 30-70%, and the light was 12 hours. The test animals were randomly divided into 25 groups.
[0021] Test product: Panax notoginseng saponins, commercially available, the content should not be less than 55.0%;
[0022] Tanshinone II A Sodium sulfonate, commercially available, the content should not be less than 97.0%;
[0023] Panax notoginseng saponins injection: 150mg;
[0024] Tanshinone II A Sodium sulfonate injection: 40mg;
[0025] Panax notoginseng saponins and tanshinone II A Sodium sulfonate compatibility injection, self-made;
[0026] See Tabl...
experiment example 2
[0032] Experimental example 2: anti-platelet aggregation effect
[0033] Experimental animals: rats, 60, weighing 200g±10g;
[0034] Test product: Panax notoginseng saponins, commercially available, the content should not be less than 55.0%;
[0035] Tanshinone II A Sodium sulfonate, commercially available, the content should not be less than 97.0%;
[0036] Panax notoginseng saponins injection: 150mg;
[0037] Tanshinone II A Sodium sulfonate injection: 40mg;
[0038] Composition injection 1: self-made, Panax notoginseng saponins: Tanshinone II A Sulfonic acid=120:12mg;
[0039] Composition injection 2: self-made, Panax notoginseng saponins: Tanshinone II A Sulfonic acid=120:30mg;
[0040] Composition injection 3: self-made, Panax notoginseng saponins: Tanshinone II A Sulfonic acid=120:240mg.
[0041] Experimental method: Rats were randomly divided into 6 groups, 10 in each group, respectively control group, Panax notoginseng sapo...
experiment example 3
[0047] Experimental Example 3: Effects on Experimental Arterial Thrombosis in Rats
[0048] Experimental animals: Wistar rats, 40, weighing 200g±10g;
[0049] Test product: Composition injection, self-made, combining Panax notoginseng saponins and Tanshinone II A Sodium sulfonate (calculated as dry product, containing C 19 h 17 NaO 6 S not less than 97.0%) were formulated into composition 1, composition 2 and composition 3 according to the weight ratio of 1:0.1, 1:0.25 and 1:2 respectively.
[0050] Experimental method: Wistar rats were taken and randomly divided into groups according to body weight, with 10 rats in each group. The rats in the control group were intravenously injected with physiological saline; the administration group was intravenously injected with 2 mg / kg of the composition injection. During the experiment, intraperitoneal injection of 20% urethane 1g / kg was anesthetized, the supine position was fixed, and the common carotid artery was separated. The s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com