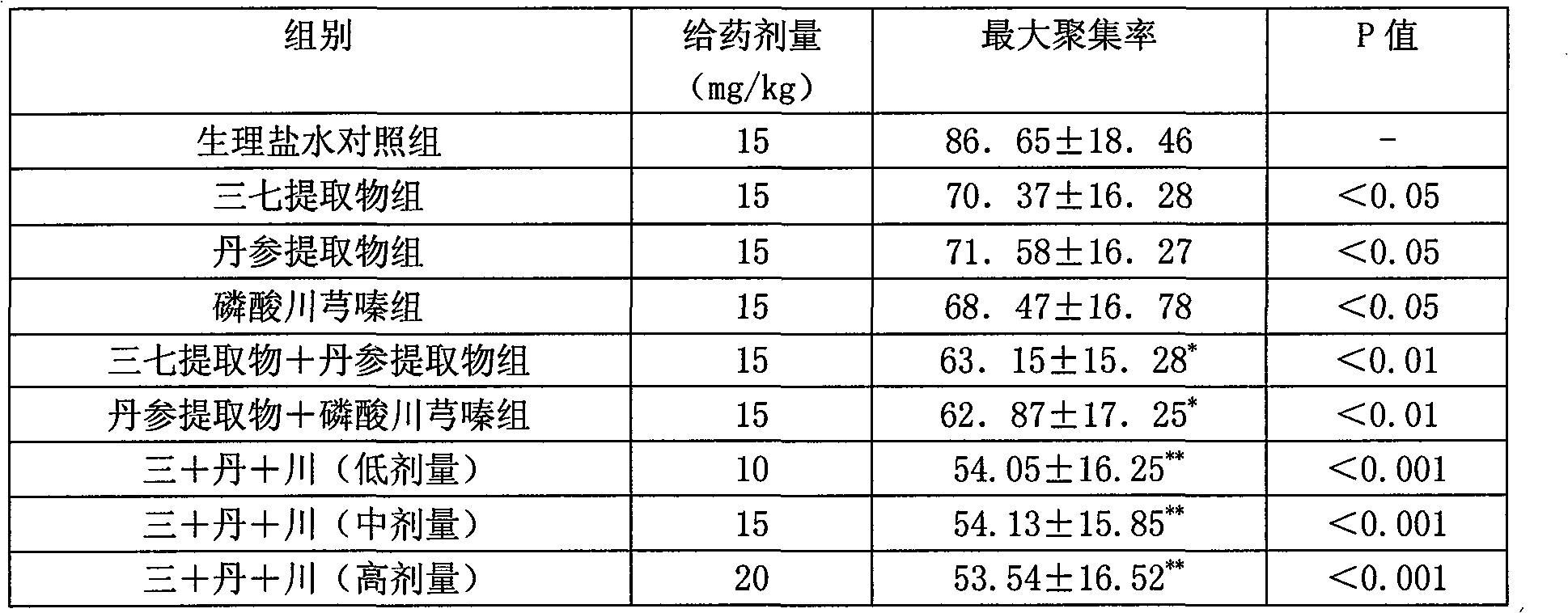
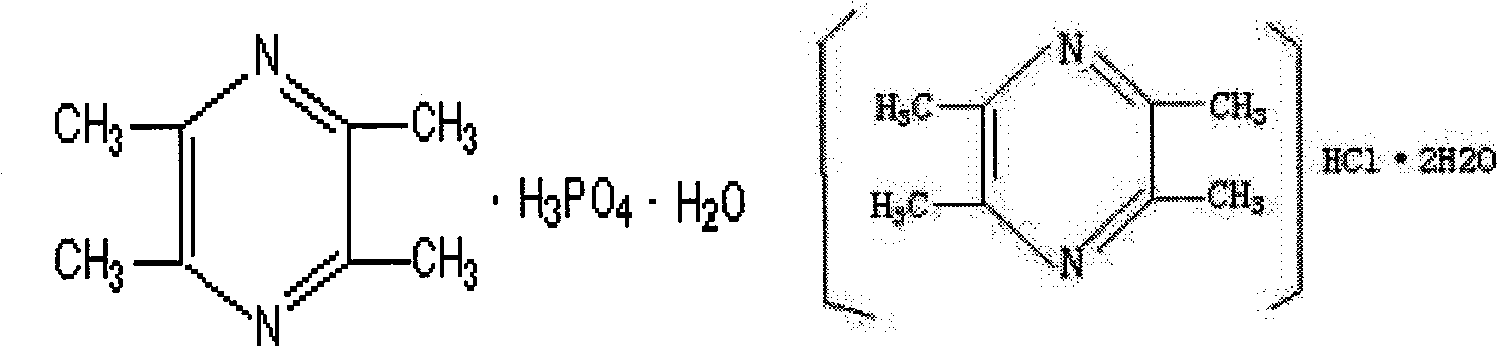Pharmaceutical composition comprising notoginseng extract, Danshen extract and ligustrazine
A technology of extracts and compositions, applied in the field of preparations containing the pharmaceutical composition and its preparation, to achieve the effects of reduced dosage, high safety, and avoidance of drug loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0030] Experimental example 1 Optimum preparation process of Panax notoginseng extract
[0031] Take the notoginseng medicinal material, crush it into a coarse powder, add ethanol to reflux and extract 3 times, each time for 3 hours, filter, recover the ethanol from the filtrate until it has no alcohol smell, add water to an appropriate amount (each 1m1 is equivalent to 2g of the original medicinal material), and refrigerate for 24 hours. Filtrate, add the filtrate to the treated weakly polar macroporous resin column, first elute with 2 times the column volume of water, then elute with 4 to 5 times of 80% ethanol, collect the eluate, and recover ethanol And vacuum drying, that is.
experiment example 2
[0032] Experimental Example 2: Determination of the content of Panax notoginseng total saponins in Panax notoginseng extract:
[0033] Preparation of Reference Substance Solution Take 10 mg of the reference substance of Panax notoginseng saponins, vacuum-dry it at 60°C for 2 hours, weigh it accurately, put it in a 25ml measuring bottle, add methanol to dissolve and dilute to the mark, shake well, filter with dry filter paper, Precisely measure 5ml of the continued filtrate, put it in a 25ml measuring bottle, add methanol to dilute to the mark, shake well, and you get it.
[0034] Preparation of the test solution Take 15 mg of this product, accurately weigh it, put it in a 25ml measuring bottle, add methanol to dissolve and dilute to the mark, shake well, filter with dry filter paper, accurately measure 5ml of the subsequent filtrate, and put it in a 25ml measuring bottle In, add methanol to dilute to the mark, shake well, that is.
[0035] Determination method Precisely mea...
experiment example 3
[0036] Experimental example 3 Salvia miltiorrhiza extraction process screening test
[0037] 1. Water extraction process screening test
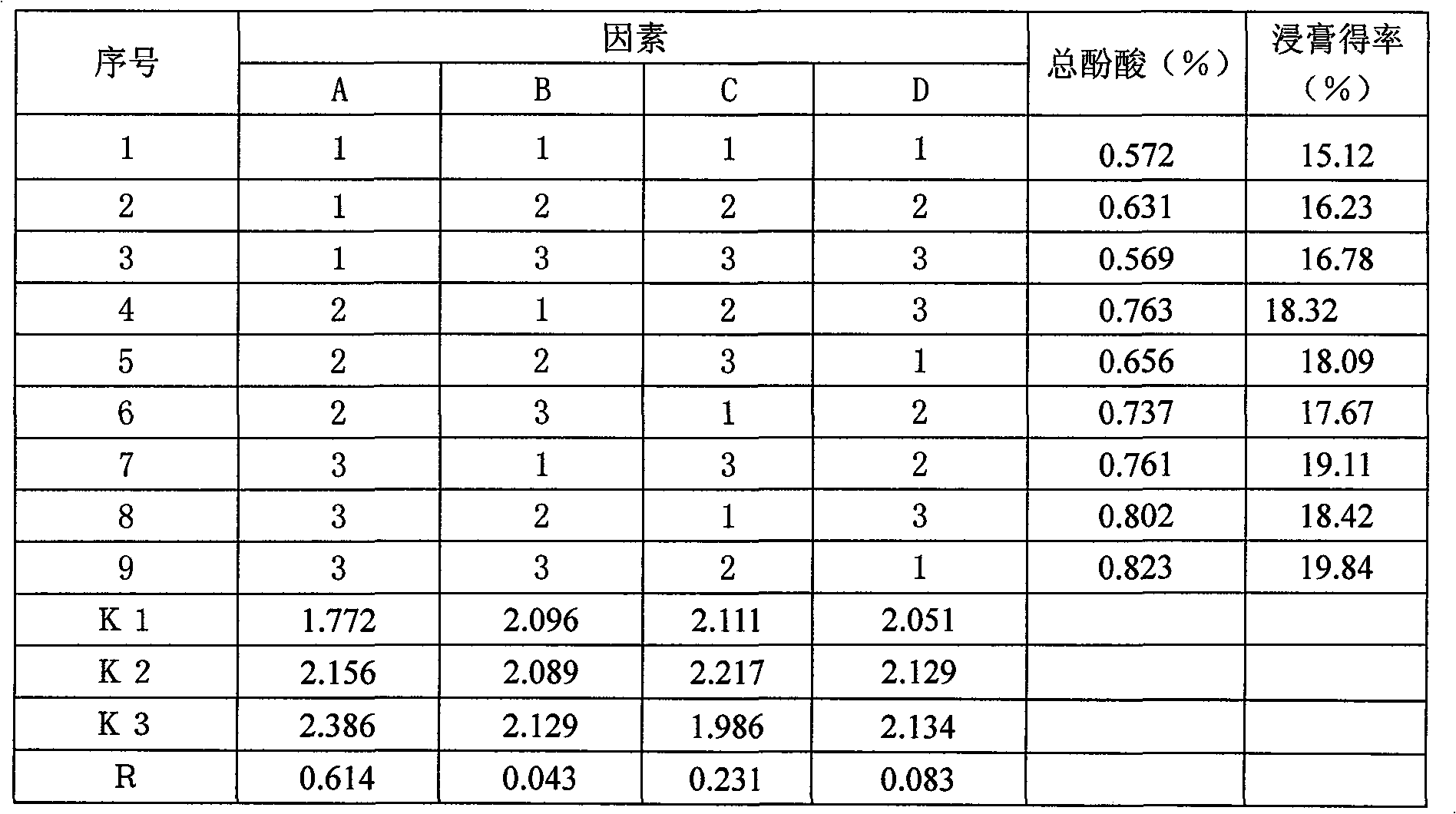
[0038] The amount of water added, extraction time and extraction times had a great influence on the extraction results, so the content of total phenolic acids in salvia miltiorrhiza and the yield of the extract were taken as indicators, and the influence of the three factors on the extraction results was investigated by orthogonal test. The factors and level design are shown in Table 2.
[0039] Table 2 Level table of factors investigated in alcohol extraction process of Danshen
[0040] level / factor
[0041] Test method Weigh 100g of Salvia miltiorrhiza, a total of 9 parts, extract according to the requirements of each column number in Table 2, combine the extracts, filter, concentrate the filtrate and dilute to 500ml, and set aside.
[0042] Content Determination of Salvianolic Acid
[0043] Preparation of reference substanc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com