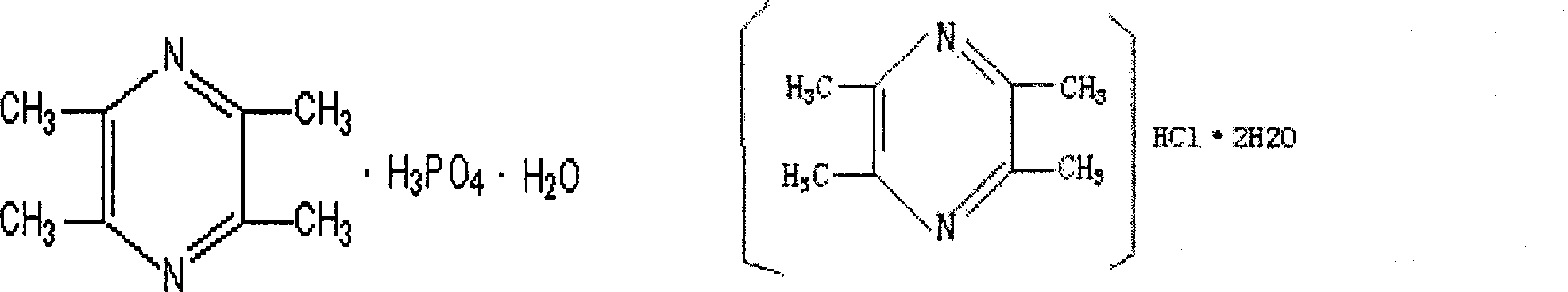
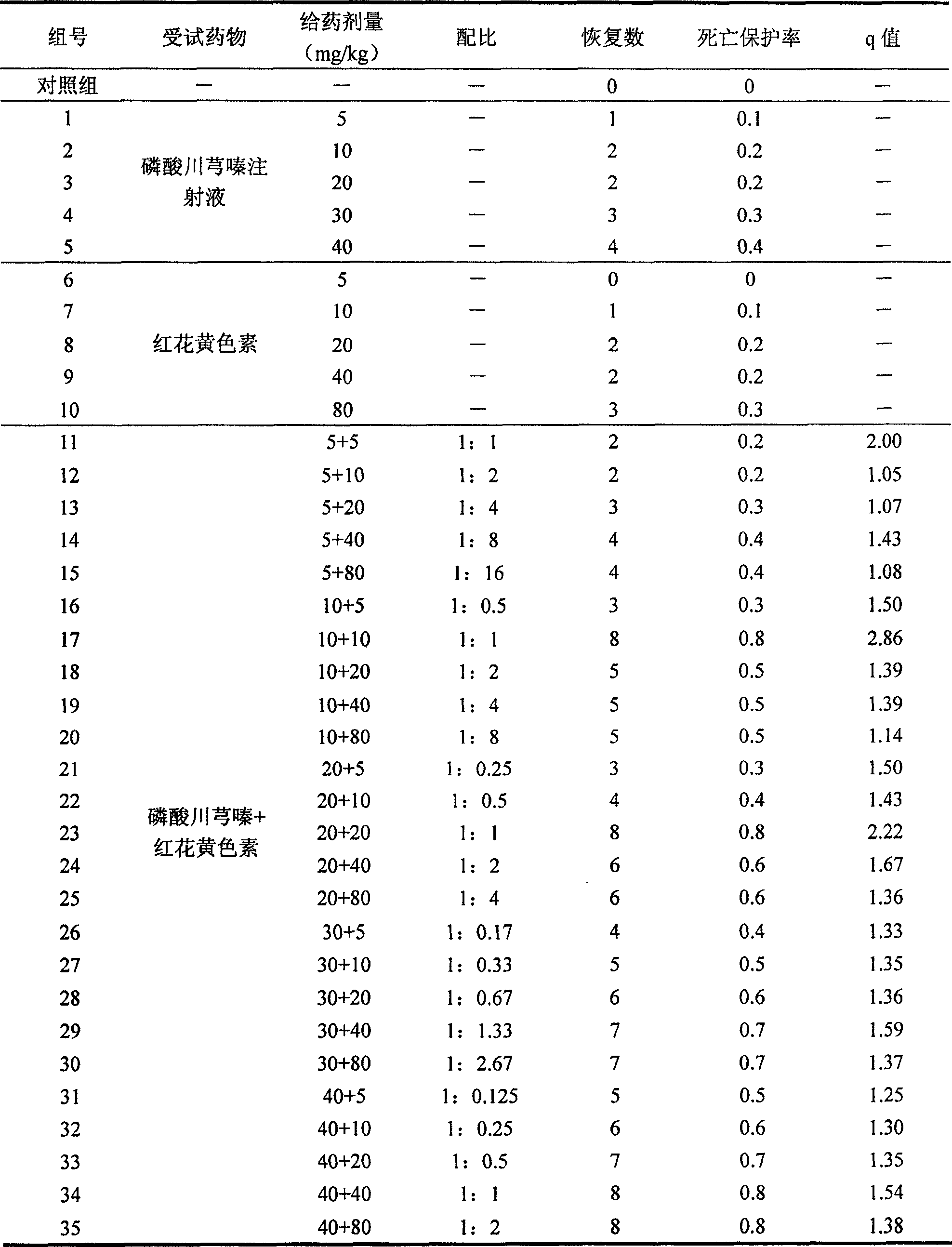
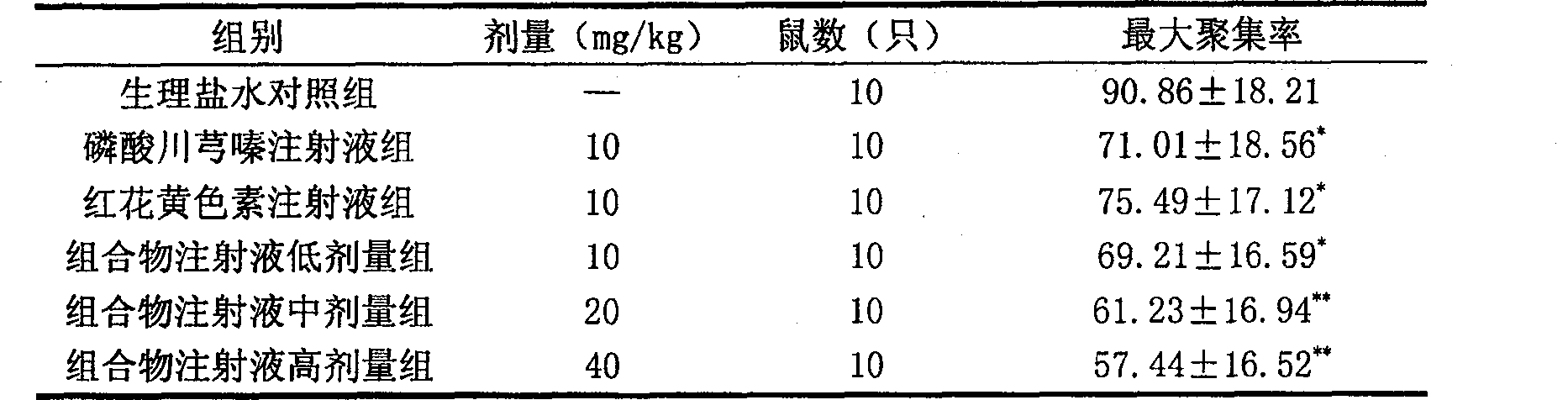
Pharmaceutical composition of ligustrazine and carthamin yellow
A technology of safflower yellow pigment and composition, which is applied in the field of medicine to achieve the effects of improved drug efficacy, less impurities, and uniform and stable drug quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Embodiment 1 Preparation of the aqueous injection of the composition
[0100] prescription:
[0101] Ligustrazine Phosphate 50g
[0102] Safflower Yellow 50g
[0103] Add water for injection to 2000ml
[0104] A total of 1000 sticks were prepared
[0105] Preparation Process:
[0106] 1) Dispose of the pipes and containers used for liquid preparation one day in advance, and rinse them with fresh water for injection before use.
[0107] 2) Take water for injection with a dosing volume of 80%, add ligustrazine phosphate and safflower yellow in the prescribed volume, heat and stir to dissolve completely.
[0108] 3) Add water for injection to the full amount.
[0109] 4) Add activated carbon for needles with a dosing volume of 0.1%, heat and stir for 15 minutes.
[0110] 5) Decarburization by sand filter stick filtration. Measure and adjust the pH of the solution.
[0111] 6) Fine filtration through a 0.45um microporous membrane.
[0112] 7) Check the clarity o...
Embodiment 2
[0117] Embodiment 2 Preparation of powder injection of this composition
[0118] prescription:
[0119] Ligustrazine Phosphate 50g
[0120] Safflower Yellow 50g
[0121] Mannitol 300g
[0122] Add sterile water for injection to 3000ml
[0123] A total of 1000 sticks were prepared
[0124] Preparation Process:
[0125] 1) First, aseptically treat the containers used for liquid preparation, antibiotic glass bottles, rubber stoppers, etc.
[0126] 2) Weigh the raw and auxiliary materials according to the prescription quantity.
[0127] 3) Take sterile water for injection with a dosing volume of 80%, add ligustrazine phosphate and safflower yellow into it, heat and stir to dissolve completely. Then add mannitol, heat and stir to dissolve completely, and add sterile water for injection to the full amount.
[0128] 4) Add activated carbon for needles with a dosing volume of 0.1%, heat and stir for 15 minutes.
[0129] 5) Decarburization by sand filter stick filtration. M...
Embodiment 3
[0135] Embodiment 3 The preparation of this composition sodium chloride injection
[0136] prescription:
[0137] Ligustrazine Phosphate 50g
[0138] Safflower Yellow 50g
[0139] Sodium chloride 900g
[0140] Add water for injection to 100000ml
[0141] A total of 1000 bottles were prepared
[0142] Preparation Process:
[0143] 1) Dispose of the pipes and containers used for liquid preparation the day before, and rinse them with fresh water for injection before use.
[0144]2) Add ligustrazine phosphate and safflower yellow into water for injection with a dosing volume of 20%, heat and stir to dissolve completely. Dissolve the sodium chloride completely with water for injection with a dosing volume of 40%.
[0145] 3) Combine the two solutions and add water for injection to the full amount.
[0146] 4) Add activated carbon for needles with a dosing volume of 0.1%, heat and stir for 15 minutes.
[0147] 5) Decarburization by sand filter stick filtration. Measure a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com