Paclitaxel liposome and preparation method thereof
A paclitaxel and liposome technology, applied in the field of medicine, can solve problems such as side effects, water insolubility of paclitaxel, and achieve the effects of reducing toxic and side effects, enhancing anti-tumor effect, and long duration of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
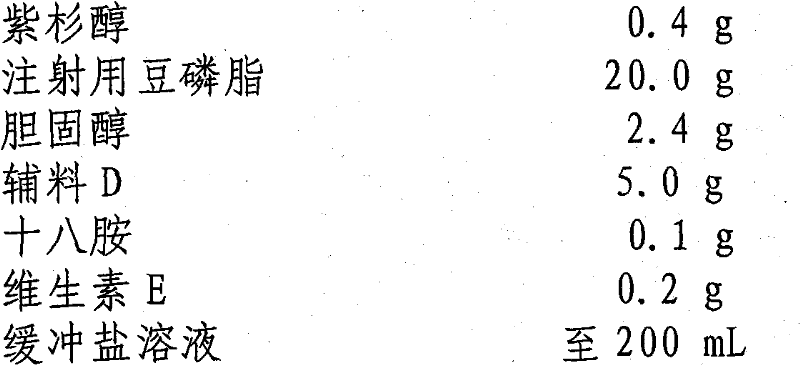
[0028] prescription:
[0029] Paclitaxel 0.4g
[0030] Soybean Lecithin for Injection 20.0g
[0031] Cholesterol 2.4g
[0032] Excipient D 5.0g
[0033] Octylamine 0.1g
[0034] Vitamin E 0.2g
[0035] Buffered saline to 200mL
[0036] Preparation Process:
[0037]Dissolve the prescribed amount of paclitaxel, soy lecithin for injection, cholesterol, excipient D, and vitamin E in an organic solvent and pour it into a pear-shaped bottle, then add a small amount of buffered salt solution containing stearylamine, ultrasonically make it into a uniform milk, and use a rotary evaporator. The organic solvent was removed under reduced pressure to form a gel, and the mixture was vortexed and distilled under reduced pressure at intervals to obtain a white homogeneous liposome solution, which was then adjusted to 200 mL with a buffer solution. Then, the pyrogen is removed by granulation through a pressurized membrane (0.22 μm), and then it is divided into vials for freeze-drying to...
Embodiment 2
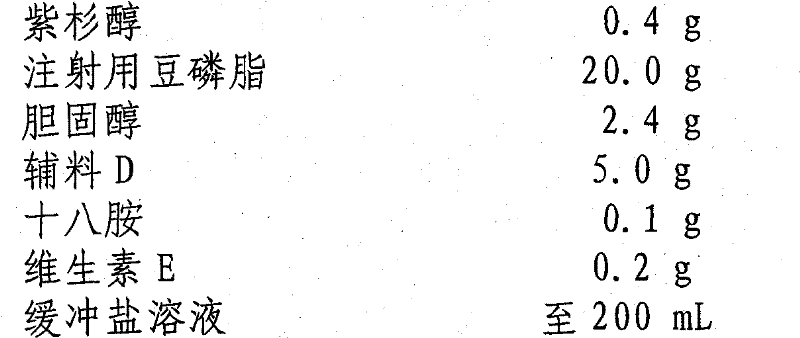
[0039] prescription:
[0040] Paclitaxel 0.4g
[0041] Soybean Lecithin for Injection 20.0g
[0042] Cholesterol 2.4g
[0043] Excipient D 5.0g
[0044] Octylamine 0.1g
[0045] Vitamin E 0.2g
[0046] Buffered saline to 200mL
[0047] Preparation Process:
[0048] Dissolve the prescribed amount of paclitaxel, soy lecithin for injection, cholesterol, excipient D, and vitamin E in an organic solvent and pour it into a pear-shaped bottle, then add a small amount of buffered salt solution containing stearylamine, ultrasonically make it into a uniform milk, and use a rotary evaporator. The organic solvent was removed under reduced pressure to form a gel, and the mixture was vortexed and distilled under reduced pressure at intervals to obtain a white homogeneous liposome solution, which was then adjusted to 200 mL with a buffer solution. Then, the pyrogen is removed by granulation through a pressurized membrane (0.22 μm), and the pellets are divided into ampoules for freezin...
Embodiment 3
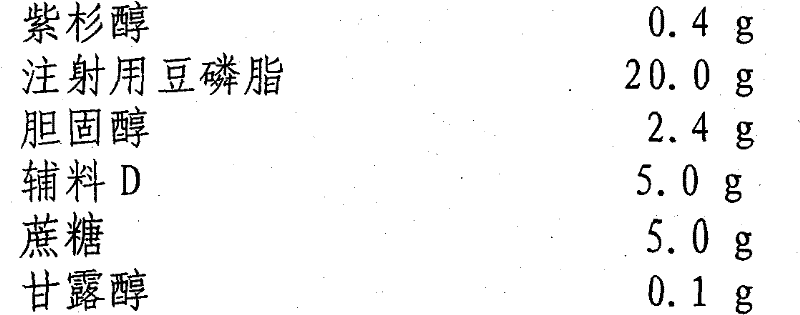
[0050] prescription:
[0051] Paclitaxel 0.4g
[0052] Soybean Lecithin for Injection 20.0g
[0053] Cholesterol 2.4g
[0054] Excipient D 5.0g
[0055] Sucrose 5.0g
[0056] Mannitol 0.1g
[0057] Octylamine 0.1g
[0058] Vitamin E 0.2g
[0059] Buffered saline to 200mL
[0060] Preparation Process:
[0061] Dissolve the prescribed amount of paclitaxel, bean lecithin for injection, cholesterol, excipient D, and vitamin E in an organic solvent and pour it into a pear-shaped bottle, spin and evaporate the organic solvent to dryness in a water bath at 30 rpm and 60 °C, and make it in a glass bottle. A dry film was formed on the wall, and the prescribed amount of sucrose and mannitol were dissolved in 200 mL of buffered saline solution, poured into a pear-shaped bottle, and rotated at 40 rpm at room temperature (25 °C) for hydration until the surface of the bottle was dry. The membrane was completely eluted and the resulting crude suspension was hydrated at room temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com