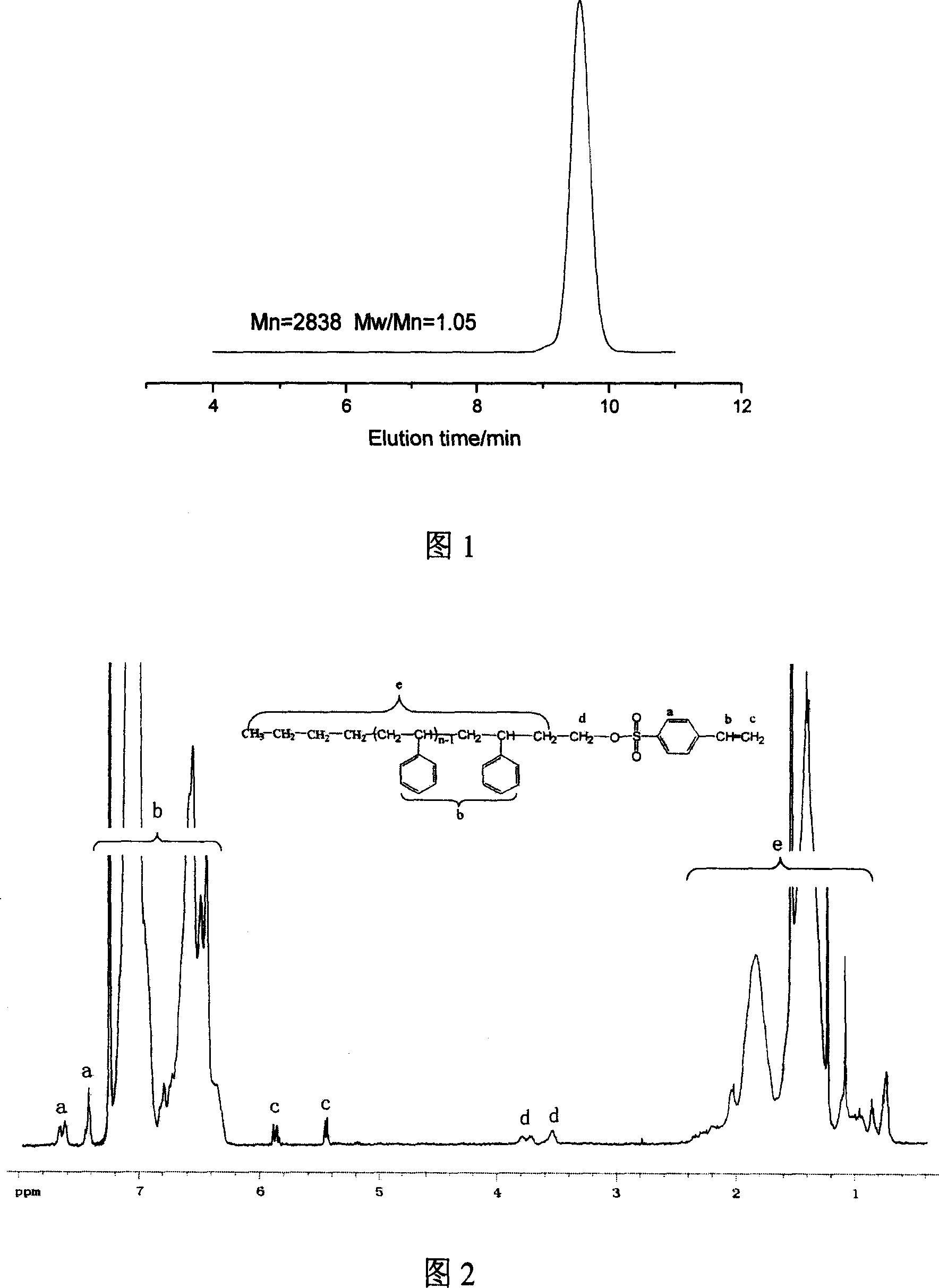
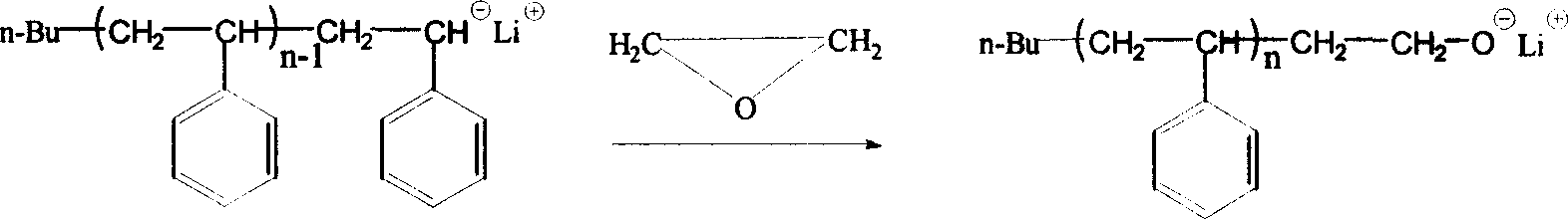
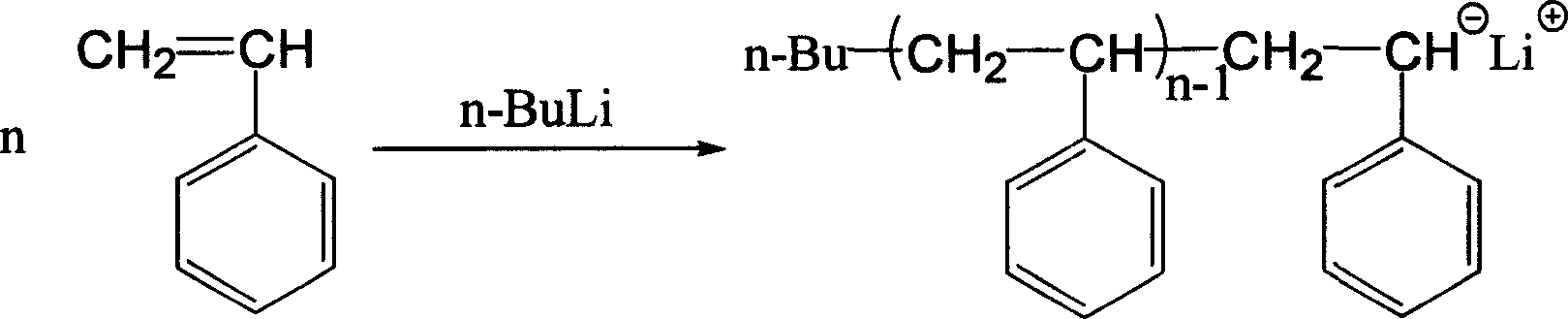Method of synthesizing macromolecule monomer based on P-vinylbenzene sulfuryl chloride end sealing agent
A technology of vinylbenzenesulfonyl chloride end-capping agent and macromonomer, which is applied in the field of polymer chemistry, can solve the problems of high double bond content and harsh conditions, and achieve the effect of high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Nitrogen gas-vacuumization-gas lamp baking bottle-nitrogen gas, repeat this 3 times, and continue to nitrogen gas to maintain positive pressure in the reaction bottle. Add 28ml of cyclohexane into the reactor with a pouring tube, then add 2.2ml of styrene and 0.5ml of tetrahydrofuran sequentially with a syringe, and start stirring. Slowly drop n-butyllithium initiator into the reactor at a certain temperature to turn the system into orange-yellow color and keep it for 1 min without fading. Then inject 1.85ml of n-butyllithium solution (0.38mol / L) quickly once at a time, and the solution is orange red of carbanion at this time. Put it into a 50°C water bath and continue the reaction for 0.5 hours. That is, the activity of polystyrene long chain.
[0024] Under nitrogen protection, feed ethylene oxide gas into the polymerization bottle until the orange-red color of the solution turns pale yellow, and react in a water bath at 25°C for half an hour.
[0025] 28 ml of tet...
Embodiment 2
[0028] Nitrogen gas-vacuumization-gas lamp baking bottle-nitrogen gas, repeat this 3 times, and continue to nitrogen gas to maintain positive pressure in the reaction bottle. Add 30 ml of cyclohexane into the reactor with a pouring tube, then add 3 ml of styrene and 0.5 ml of tetrahydrofuran sequentially with a syringe, and start stirring. Slowly drop n-butyllithium initiator into the reactor at a certain temperature to turn the system into orange-yellow color and keep it for 1 min without fading. Then inject 1.5ml of n-butyllithium solution (0.45mol / L) quickly once at a time, and the solution is orange red of carbanion at this moment. Put it into a 50°C water bath and continue the reaction for 0.5 hours. That is, the activity of polystyrene long chain.
[0029] Under the protection of nitrogen, ethylene oxide gas was passed into the polymerization bottle until the orange-red color of the solution turned pale yellow, and the polymerized polystyrene was reacted in a water bat...
Embodiment 3
[0033] Take the three reaction flasks labeled ①②③, and follow the steps below to synthesize reactive polystyrene and react with ethylene oxide.
[0034] Nitrogen gas-vacuumization-gas lamp baking bottle-nitrogen gas, repeat this 3 times, and continue to nitrogen gas to maintain positive pressure in the reaction bottle. Add 30 ml of cyclohexane into the reactor with a pouring tube, then add 3 ml of styrene and 0.5 ml of tetrahydrofuran sequentially with a syringe, and start stirring. Slowly drop n-butyllithium initiator into the reactor at a certain temperature to turn the system into orange-yellow color and keep it for 1 min without fading. Then inject 1.5ml of n-butyllithium solution (0.87mol / L) rapidly once at a time, and the solution is orange red of carbanion at this moment. Put it into a 50°C water bath and continue the reaction for 0.5h. That is, the activity of polystyrene long chain.
[0035] Under the protection of nitrogen, feed ethylene oxide gas into the polymer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com