Catalyst for preparing heavy hydrocarbon with synthetic gas and its prepn process
A catalyst, heavy hydrocarbon technology, applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of low octane number and low utilization value, and achieve increased added value and low cost. , highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Catalyst A is composed of Co and SiO 2 , The weight percentage of Co element is 15%, SiO 2 The specific surface area is 380m 2 / g, the average pore diameter is 10nm, and the pore volume is 0.91ml / g. The catalyst is prepared according to the following steps:
[0020] a) Impregnate 12.75g of the above SiO with 18ml of cobalt nitrate solution containing the required cobalt content 2 ;
[0021] b) Drying the catalyst obtained in step a) at 393K for 6 hours;
[0022] c) calcining the catalyst obtained in step b) at 623K for 6 hours.
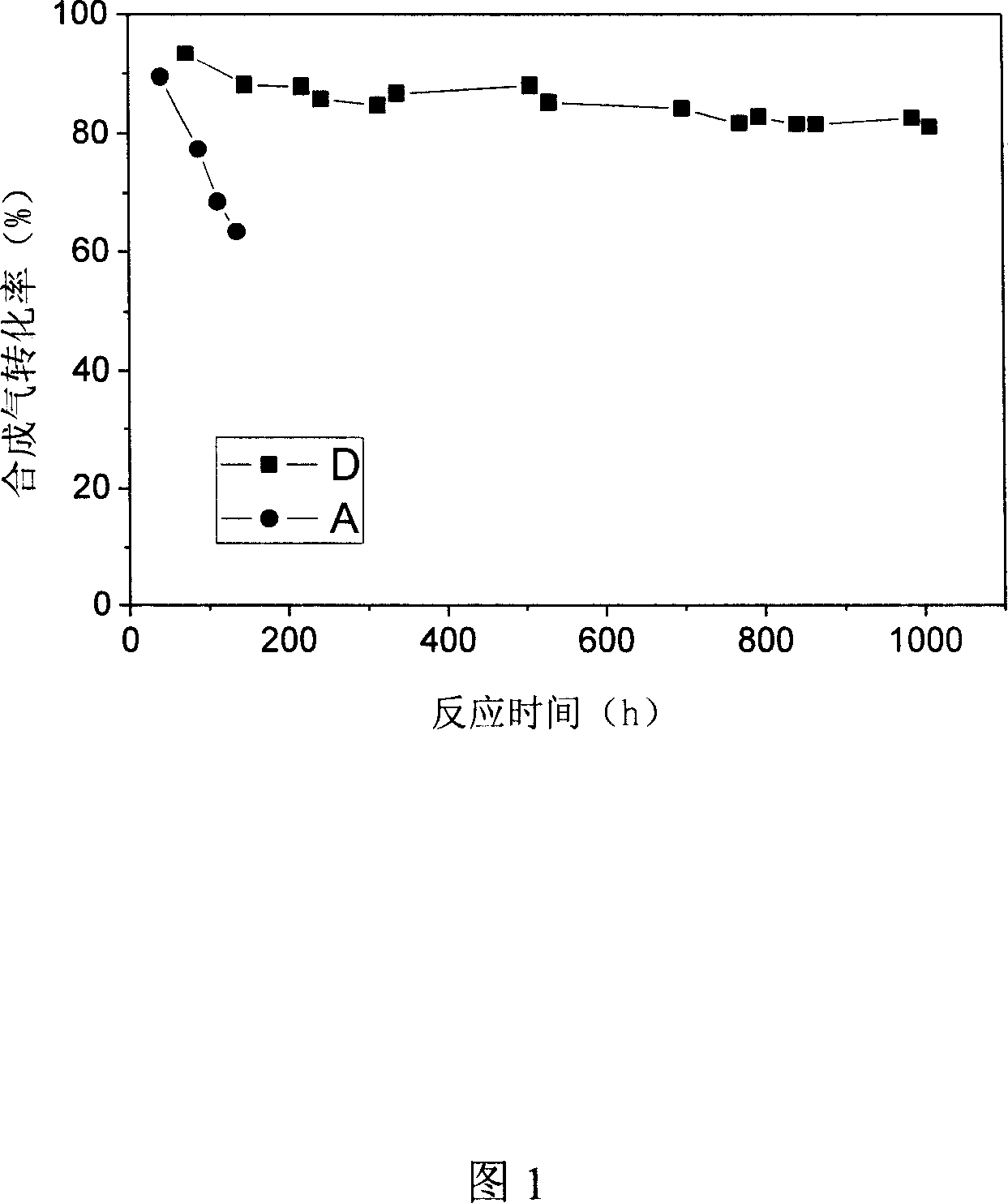
[0023] Catalyst A was activated for 2 hours under 673K hydrogen atmosphere before use. The reaction conditions of catalyst A are: reaction temperature is 493K, reaction pressure is 2.0Mpa, and synthesis gas space velocity is 500h -1 , The volume ratio of hydrogen to carbon monoxide in the synthesis gas is 2.
Embodiment 2
[0025] Catalyst B is composed of Co and SiO 2 , The weight percentage of Co element is 15%, SiO 2 The specific surface area is 286m 2 / g, the average pore diameter is 13nm, and the pore volume is 0.98ml / g. The catalyst is prepared according to the following steps:
[0026] a) Impregnate 12.75g of the above SiO with 18ml of cobalt nitrate solution containing the required cobalt content 2 ;
[0027] b) Drying the catalyst obtained in step a) at 393K for 6 hours;
[0028] c) calcining the catalyst obtained in step b) at 623K for 6 hours.
[0029] The reduction conditions and reaction conditions of the catalyst B are the same as those of the catalyst A.
Embodiment 3
[0031] Catalyst C is composed of Co, Zr and SiO 2 , Co element weight percentage is 15%, Zr element weight percentage is 0.5%, SiO 2 The specific surface area is 286m 2 / g, the average pore diameter is 13nm, and the pore volume is 0.98ml / g. The catalyst is prepared according to the following steps:
[0032] a) Impregnate 12.75g of the above SiO with 18ml of zirconium nitrate solution containing the required zirconium content 2 ;
[0033] b) Drying the catalyst obtained in step a) at 393K for 6 hours;
[0034] c) calcining the catalyst obtained in step b) at 1073K for 6 hours;
[0035] d) Immerse the catalyst obtained in step c) above with 18 ml of cobalt nitrate solution containing the required cobalt content;
[0036] e) drying the catalyst obtained in step d) at 393K for 6 hours;
[0037] f) The catalyst obtained in step e) is calcined at 623K for 6 hours.
[0038] The reduction conditions and reaction conditions of the catalyst C are the same as those of the catalyst A.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com