New type of phosphonate-nucleotide compound
A compound and phosphonate technology, applied in the field of chemistry, can solve the problems of low seroconversion rate, inability to absorb, and difficulties in drug resistance, and achieve good safety, high antiviral activity, and high oral absorbability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthesis of compound No.1
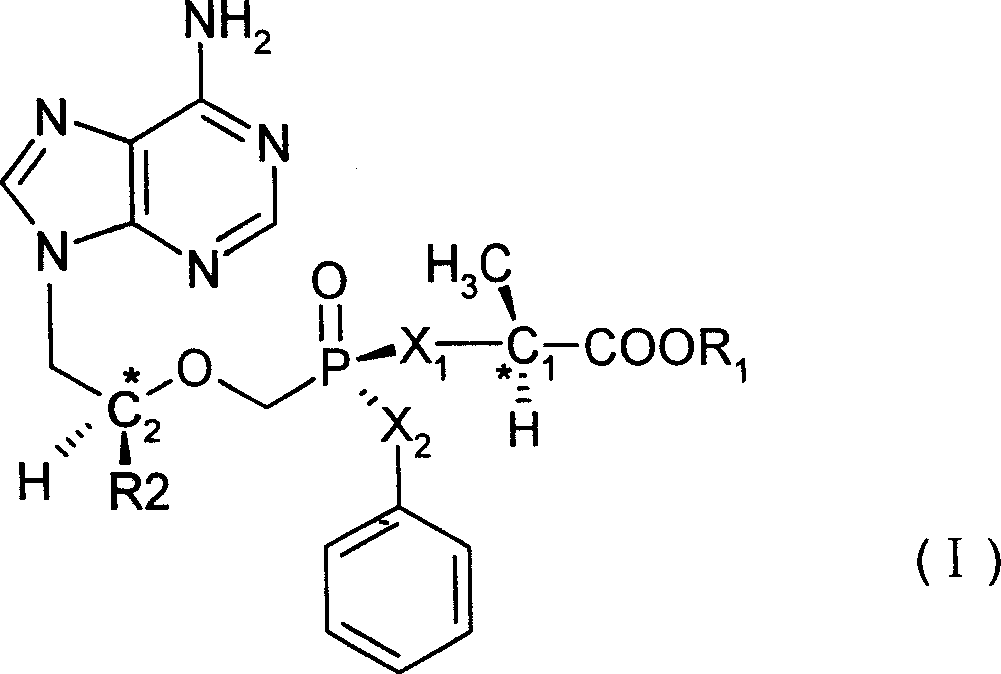
[0026] The structural formula of No.1 compound is shown in the figure below, in which the carbon atom C1 is a left-handed structure:
[0027]
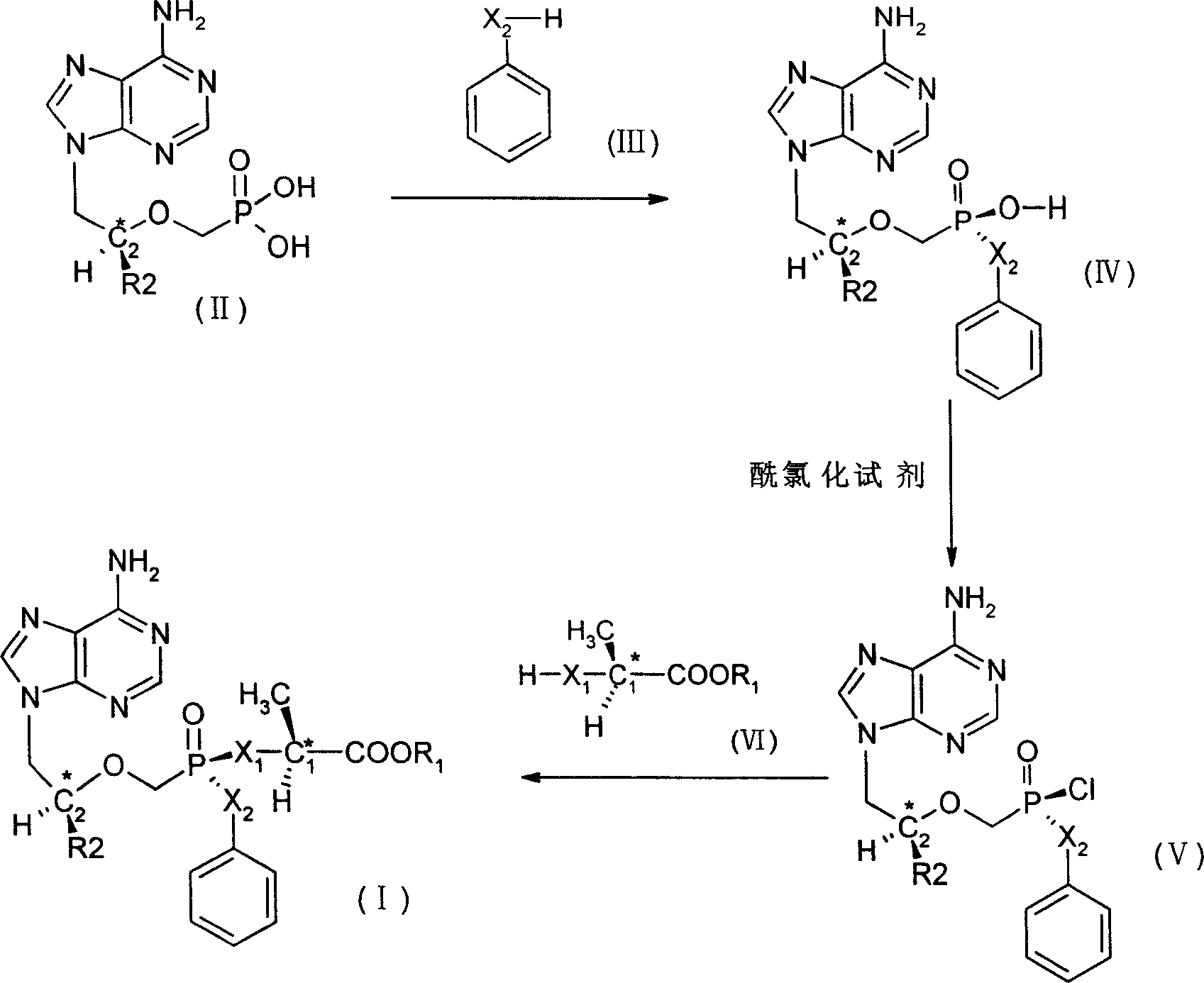
[0028]Add 10 g of 9-(2-phosphonomethoxyethyl) adenine (PMEA), 7 g of phenol, 5 g of triethylamine and 30 g of tetrahydrofuran into the reactor, and heat to reflux. Then add N, N'-carbonylbisimidazole 15g. The reflux reaction was continued for 24 hours. The reaction solution was cooled to 25°C. The reaction solution was concentrated under reduced pressure into a yellow-brown slurry, then 18 g of water was added, and the pH value was adjusted to 11 with 25% NaOH solution. Suspended solids were removed by filtration through 1.5 g of celite followed by rinsing with 3 g of water. The combined filtrates were extracted with ethyl acetate. The aqueous phase was adjusted to pH 3.1 with 37% HCl solution, and the 9-(2-phenoxyphosphonomethoxyethyl)adenine precipitate was separated by fi...
Embodiment 2
[0031] Embodiment 2: the synthesis of compound No.2
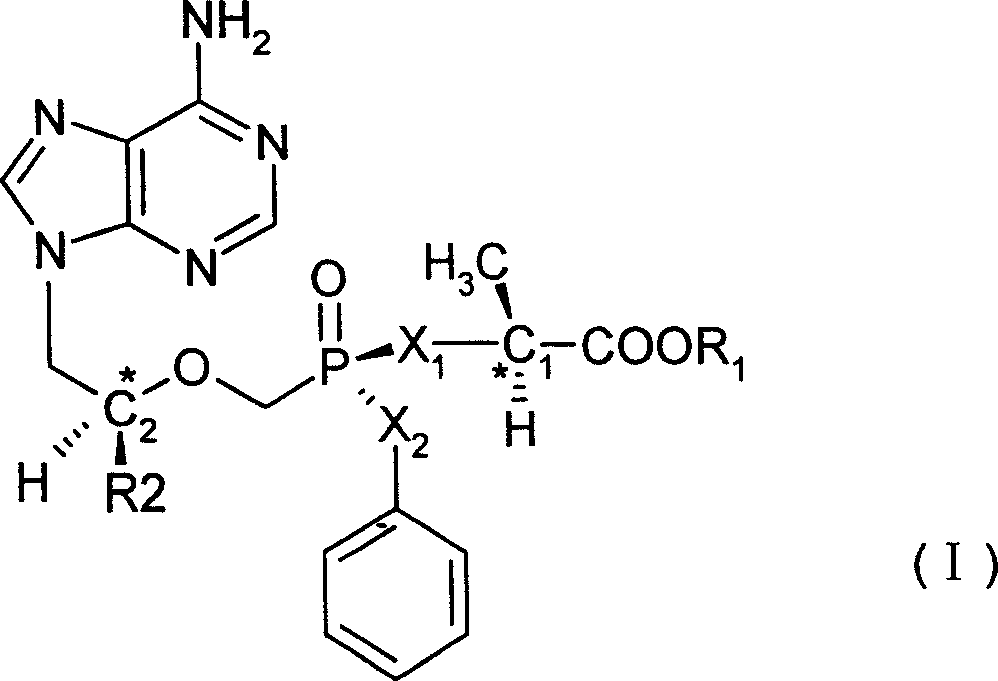
[0032] The structural formula of the No.2 compound is shown in the figure below, in which the carbon atom C1 is a left-handed structure:
[0033]
[0034] According to the same method as in Example 1, 10 g of 9-(2-phosphonomethoxyethyl) adenine (PMEA), 7 g of phenol, 5 g of triethylamine and 30 g of tetrahydrofuran were added to the reactor, and heated to reflux. Then add N, N'-carbonylbisimidazole 15g. The reflux reaction was continued for 24 hours. The reaction solution was cooled to 25°C. The reaction solution was concentrated under reduced pressure into a yellow-brown slurry, then 18 g of water was added, and the pH value was adjusted to 11 with 25% NaOH solution. Suspended solids were removed by filtration through 1.5 g of celite followed by rinsing with 3 g of water. The combined filtrates were extracted with ethyl acetate. The aqueous phase was adjusted to pH 3.1 with 37% HCl solution, and the 9-(2-phenoxypho...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap