Anti-addl antibodies and uses thereof
A technology of antibodies and ligands, applied in the direction of antibodies, anti-animal/human immunoglobulins, instruments, etc., can solve the problem of low correlation between dementia and amyloid plaques
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1: General Materials and Methods
[0089]Preparation of ADDL. ADDLs in F12 medium (Biosource, Camarillo, CA) were prepared from Aβ1-42 following established methods (Lambert et al. (2001) supra). Briefly, the Aβ1-42 peptide (American Peptide Co., Sunnyvale, CA or California Peptide Research, Inc., Napa, CA) was weighed and placed in a container capable of holding a sufficient amount of HFIP (1, 1, 1, 3, 3 , 3-hexafluoro-2-propanol) in a glass vial to bring the peptide concentration to 10 mg / mL. HFIP was added to the dried peptide, the glass vial was capped, vortexed gently to mix, and the peptide / HFIP solution was stored at room temperature for at least 1 hour. Aliquots (50 or 100 μL, containing 0.5 or 1.0 mg, respectively) of the peptide solution were dispensed into a series of 1.5 mL conical centrifuge tubes. The tubes were placed in a speedvac overnight to remove HFIP. Tubes containing dried peptide films were capped and stored at -70°C in a sealed contai...
Embodiment 2
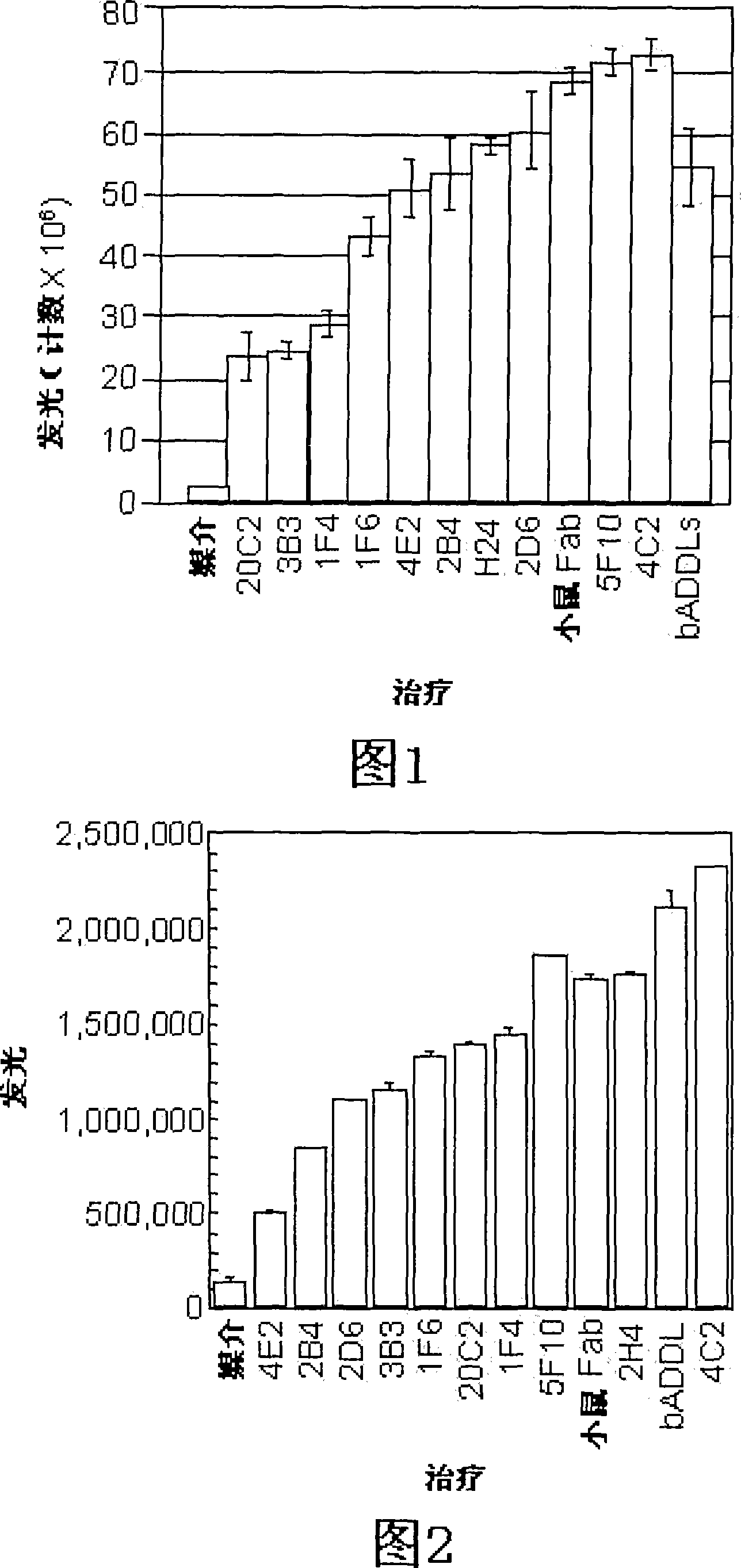
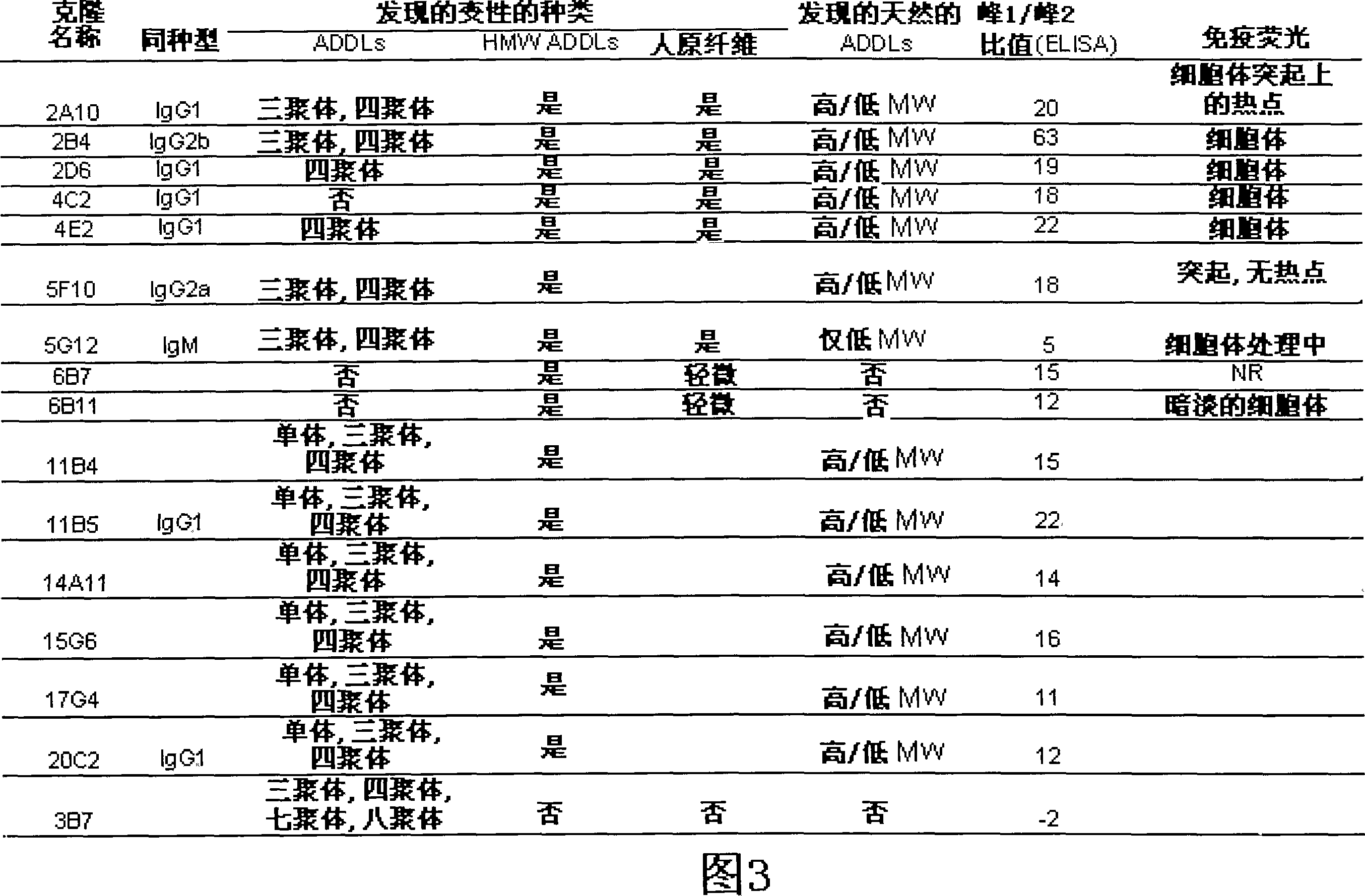
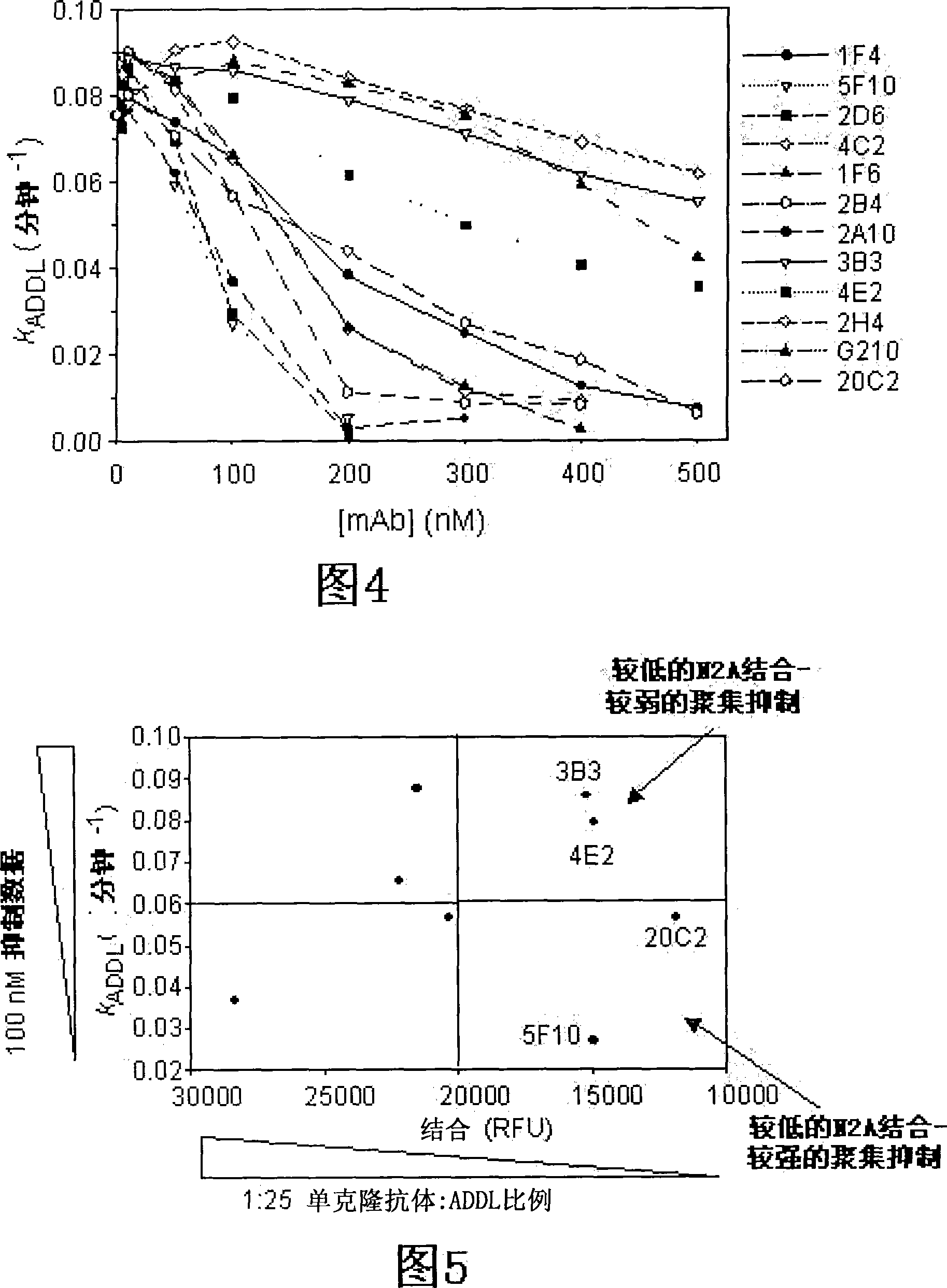
[0111] Example 2: Development and characterization of anti-ADDL antibodies
[0112] Three mice were inoculated with ADDLs (194±25 μg protein per injection) every three weeks for a total of six inoculations. Hybridomas obtained from the fusion of spleen cells from these mice with SP2 cells were cultured in 96-well plates. Supernatants from these wells were screened in dot blots and synthetic ADDLs were used to identify positive clones that could be compared to dot blots of endogenous fibrils to discern differences. Look for hybridomas that bind only synthetic ADDLs and not endogenous fibrils. To learn more about what the products of the hybridomas bind to and under what conditions, 3 western blots were performed on each positive clone: SDS-PAGE of ADDLs, native gel of ADDLs, and SDS-PAGE with endogenous fibrils . Approximately 40 clones were screened for further examination. Each clone was tested under different conditions for recognition of soluble Alzheimer's patient br...
Embodiment 3
[0116] Example 3: Immunohistochemical analysis of endogenous and synthetic ADDLs associated with cultured hippocampal cells
[0117] Cultured hippocampal cells were also analyzed to determine whether monoclonal antibodies capable of distinguishing Alzheimer's disease from control brain extracts were able to identify ADDLs (endogenous or synthetic) bound to cultured cells. Hippocampal cultures were prepared according to established protocols and allowed to grow for 3-4 weeks. Synthetic ADDLs are prepared according to standard procedures (eg, U.S. Patent 6,218,506). Endogenous ADDLs were extracted from the brains of Alzheimer's patients according to the method of Gong et al. ((2003) supra). ADDLs (100 nM in F12, or 2 mg total protein in F12) were incubated with cells for 1 hour, then washed and fixed according to standard methods. After washing, cells were incubated with 20C2, 3B7, M94, 2A10, 4E2, 2D6, 4C2, 2B4, 5F10, or 5G12 monoclonal antibody, followed by an anti-mouse seco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com