Particle and preparation containing the particle
A particle and average particle size technology, applied in the field of particles, can solve the problems of irritating dosage, difficult nasal administration, difficult micronization, etc., and achieve the effect of full effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
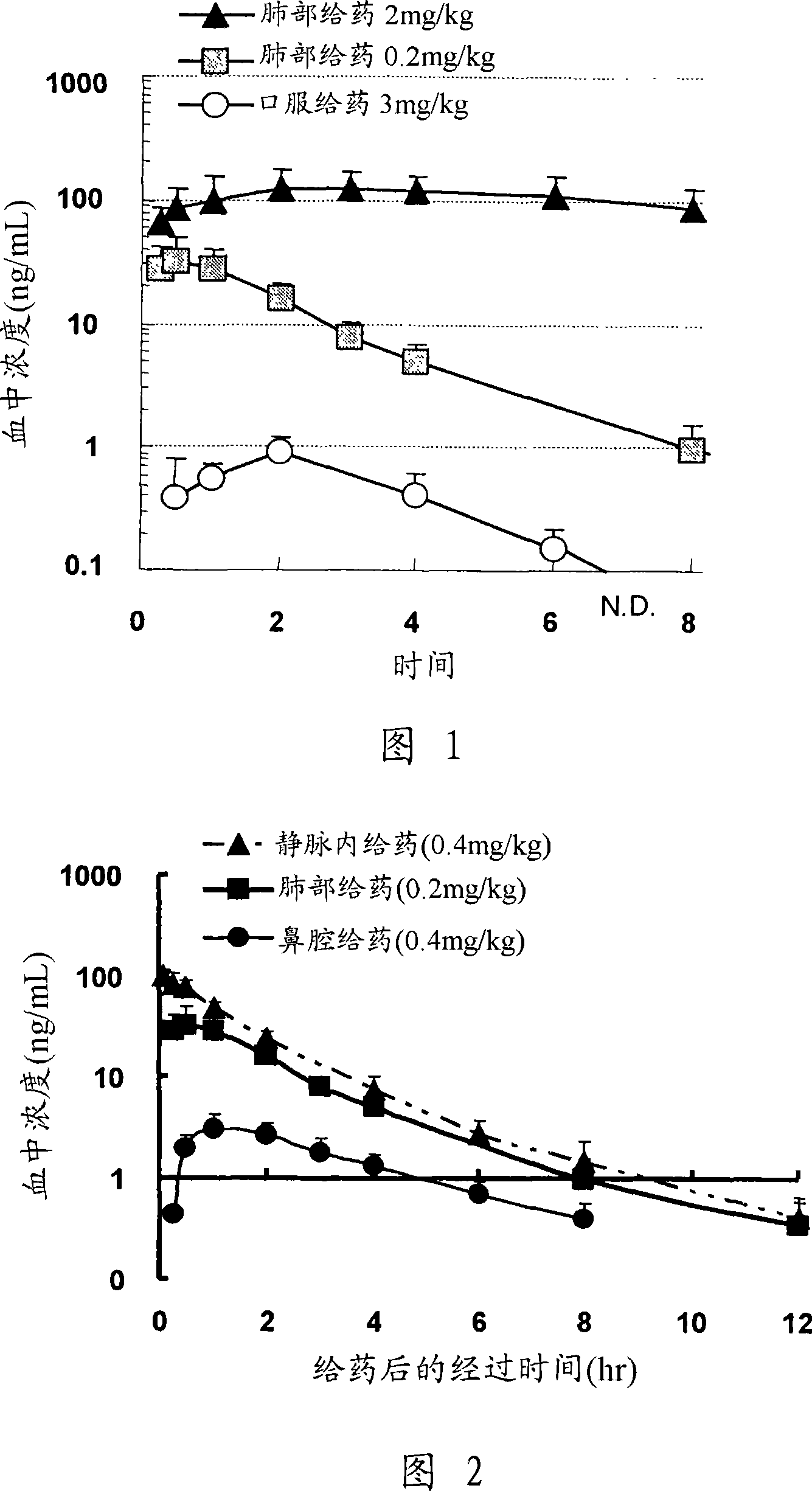
[0096] Hereinafter, the present invention will be more specifically described by way of formulation examples, comparative examples, absorption test examples, and test examples, but the present invention is not limited thereto. In the following inhalation experiments, the cascade collision classifier is the particle size evaluation device for inhalation preparations described in the 28th revision of the US Pharmacopoeia, and the basic operation method is carried out in accordance with the 28th revision of the US Pharmacopoeia. When predicting the absorption of a compound from the lungs, the lung delivery of inhaled formulations can be assessed in vitro using a cascade impactor.
[0097] In addition, a hexane solution (20 mM) of the aerosol OT was used as a dispersion medium in the measurement of the particle size. The measurement particles were put into the test tube, and 1 mL of the dispersion medium was added to make a suspension. The suspension was sonicated for 1 minute, a...
preparation example 1
[0098] Preparation Example 1: Preparation of Suspension
[0099] After dissolving HPC (hydroxypropylcellulose)-SL (150 g) and sodium dodecyl sulfate (SDS) (1.5 g) in purified water (2548.5 g), Compound 1 (300 g) was suspended therein. Then, the suspension was pulverized in water with a pulverizer to obtain a suspension for pulmonary administration (3000 g) (theoretical concentration 10% w / w, measured concentration 8.37% w / w). The average particle size of Compound 1 in the resulting suspension was 201 nm.
preparation example 2
[0100] Formulation Example 2: Preparation of Suspension
[0101] After dissolving HPC-SL (100 g) and sodium lauryl sulfate (1 g) in purified water (1699 g), compound 1 (200 g) was suspended therein. Then, the suspension was pulverized in water by a pulverizer to obtain a suspension (2000 g) of Compound 1 (theoretical concentration 10% w / w, measured concentration 9.69% w / w). The suspension (5g) was diluted with a solution (57.5g) of HPC-SL (5.556w / w%) and sodium lauryl sulfate (0.056w / w%) in purified water (94.389w / w%), A suspension for pulmonary administration is obtained. The average particle size of Compound 1 in the resulting suspension was 395 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com