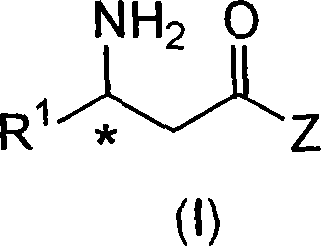
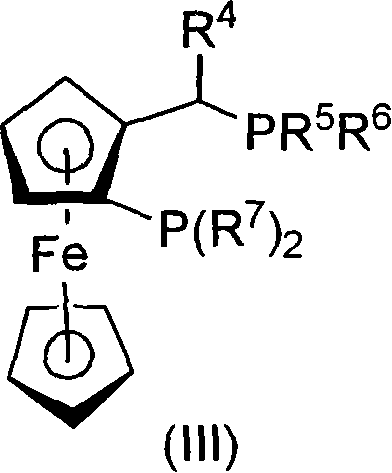
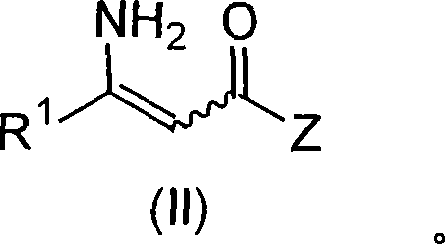
Process to chiral beta amino acid derivatives by asymmetric hydrogenation
A technology of compounds and alkyl groups, applied in the field of preparing chiral beta amino acid derivatives by asymmetric hydrogenation, can solve problems such as difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095]
[0096] (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-α]pyrazine-7(8H )- Base]-1-(2,4,5-trifluorophenyl)butan-2-amine (2-5)
[0097] 3-(Trifluoromethyl)-5,6,7,8-tetrahydro[1,2,4]triazolo[4,3-α]pyrazine, hydrochloride Preparation of (1-4)
[0098] Process 1
[0099]
[0100] Step A: Preparation of bishydrazide (1-1)
[0101] Hydrazine (20.1 g, 35% by weight in water, 0.22 mol) was mixed with 310 mL of acetonitrile. 31.5 g of ethyl trifluoroacetate (0.22 mol) were added over 60 min. The internal temperature increased from 25°C to 14°C. The resulting solution was aged at 22-25°C for 60 min. Cool the solution to 7°C. At a temperature below 16°C, 17.9 g of 50% by weight aqueous NaOH (0.22 mol) and 25.3 g of chloroacetyl chloride (0.22 mol) were added simultaneously over 130 min. When the reaction was complete, the mixture was vacuum distilled at 27-30°C and 26-27 Hg vacuum to remove water and ethanol. During the distillation, 720...
Embodiment 2
[0127] (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-α]pyrazine-7(8H )- Preparation of -1-(2,4,5-trifluorophenyl)butan-2-amine (2-5)
[0128] Under a nitrogen atmosphere, the chloro(1,5-cyclooctadiene) rhodium(I) dimer {[Rh(cod)Cl] 2} (0.074 mg, 0.15 μmol) and (R,S) tert-butyl Josiphos (0.179 mg, 0.033 μmol), ammonium formate (6.6 mg, 0.15 mmol) and ketoamide 2-3 (6.1 mg, 15 μmol) into a 1-mL reactor. Additional degassed MeOH (200 μL) was added and the mixture was stirred for 5 h at 55° C. in a pressure vessel under nitrogen. The mixture was hydrogenated under 250 psi hydrogen at 55°C for 20 h. The experimental yield was 91% and the optical purity was 95% ee as determined by HPLC.
[0129] The following high performance liquid chromatography (HPLC) conditions were used to determine the percent conversion of the product:
[0130] Column: Agilent Extend C18, 150mm×4.6mm
[0131] Eluent: Solvent A: 80 / 20% by volume Water / Methanol 10mM TRIS pH 9
[01...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap