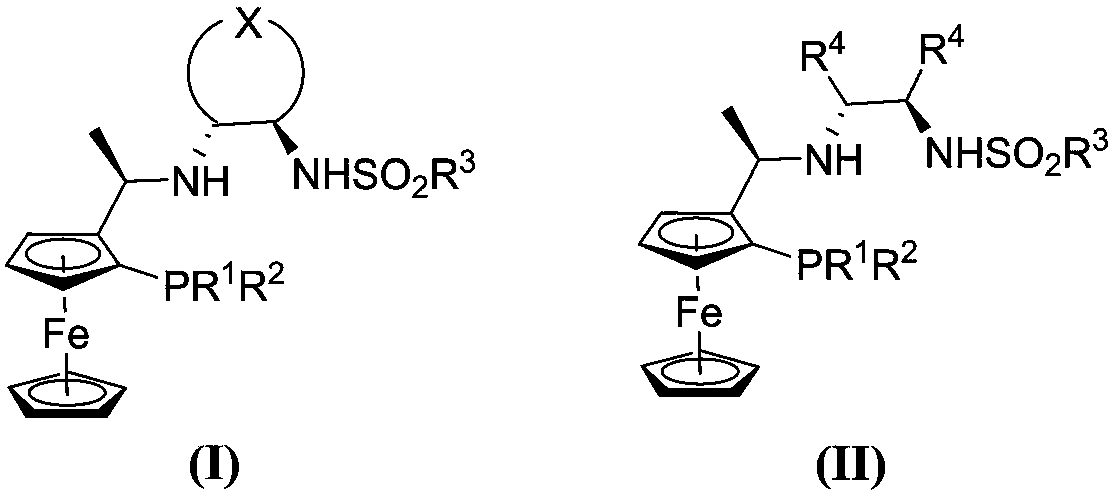
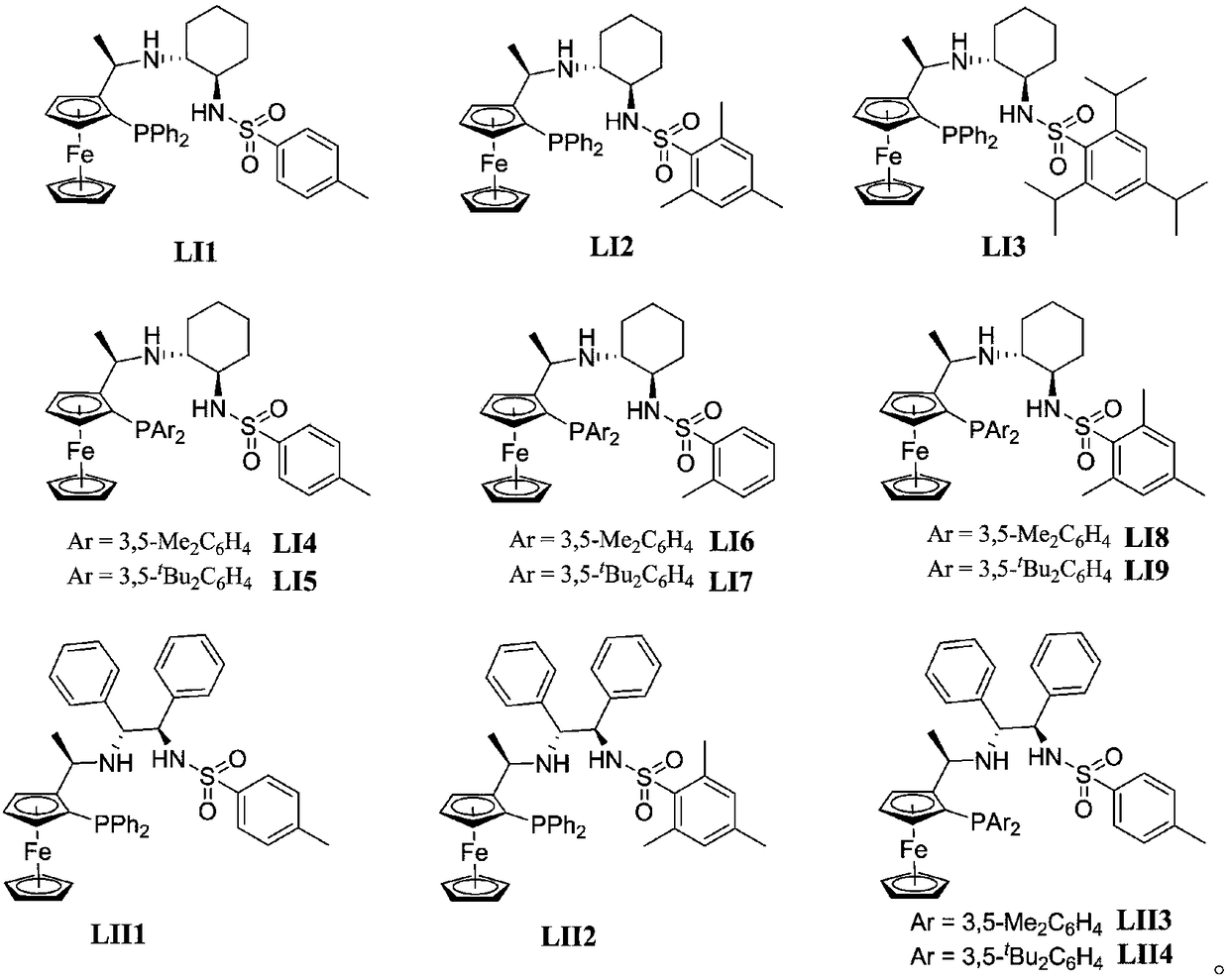
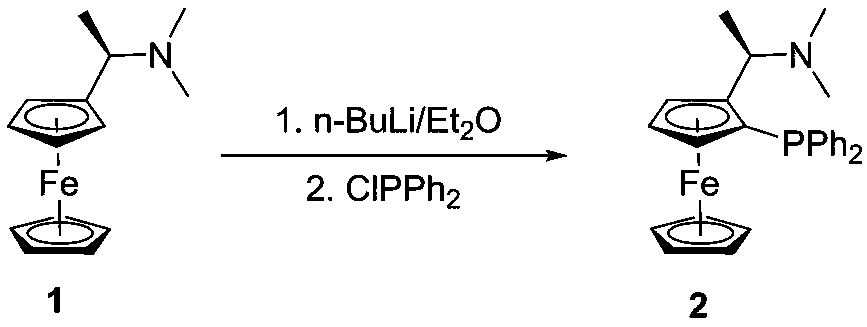A chiral nitrogen nitrogen phosphine tridentate ligand based on a ferrocene skeleton and an application thereof
A ferrocene and skeleton technology, applied in the field of chiral phosphine nitrogen tridentate ligands, can solve the problems of high price and long synthesis route of spiro ring ligands, and achieve easy purification, high industrial application value, water and air stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Synthesis of Ligand LI2
[0021]
[0022] Dissolve (S)-Ugi-amine 1 (5.14g, 20mmol) in 50mL ether, under nitrogen protection and ice-salt bath cooling, add n-butyllithium (16mL, 2.5mol / L) dropwise to the reaction system, dropwise After completion, the temperature was slowly raised to room temperature, and the reaction was stirred for 3 hours. Diphenylphosphine chloride (8.82 g, 40 mmol) was added dropwise under cooling in an ice-salt bath, and after the drop was completed, the temperature was slowly raised to room temperature, and the reaction was stirred for 12 hours. The reaction was quenched with saturated sodium bicarbonate solution, extracted with dichloromethane, dried over anhydrous sodium sulfate, concentrated and column chromatographed to obtain compound 2 (5.38 g, 61%).
[0023]
[0024] Compound 2 (4.41 g, 10 mmol) was dissolved in 8.0 mL of acetic anhydride, and reacted at 55° C. for 4 hours. After the reaction was completed, excess acetic ...
Embodiment 2
[0029] Example 2: Synthesis of Ligand LI3
[0030] The preparation method of crude product 3 is the same as that of Example 1.
[0031]
[0032] Crude products 3 (0.46 g, 0.1 mmol) and 5 (0.53 g, 0.2 mmol) were added to the reaction flask, and after replacing the nitrogen, 5 mL of methanol was added and reacted at 50° C. for 15 hours. Concentration and column chromatography gave yellow ligand LI3 (0.40 g, 51%).
[0033]
[0034] 1 H NMR (600MHz, CDCl 3 )δ7.48(m, 2H), 7.35(m, 3H), 7.15(s, 2H), 7.08(t, J=7.1Hz, 3H), 6.96(t, J=6.8Hz, 2H), 5.96( s,1H),4.50(s,1H),4.27(s,1H),4.08(m,1H),4.05(d,J=7.0Hz,1H),4.04(s,5H),4.03(d,J =6.8Hz,1H 1H),3.64(s,1H),3.02–2.92(m,1H),2.22–1.92(m,4H),1.49(t,J=10.2Hz,2H),1.39(d,J =5.4Hz, 3H), 1.28(dd, J=6.8, 5.3Hz, 6H), 1.23(d, J=6.8Hz, 6H), 1.17(d, J=6.8Hz, 6H), 1.09–0.96(m ,2H),0.14(s,1H),-0.20(d,J=11.5Hz,1H). 13 C NMR (151MHz, CDCl 3 )δ152.07,150.28,139.96,139.88,136.45,136.38,135.13,134.96,133.54,132.71,132.56,129.16,128.31,128.26,128.1...
Embodiment 3
[0035] Example 3: Synthesis of Ligand LI5
[0036]
[0037] (S)-Ugi-amine 1 (2.57g, 10mmol) was dissolved in 25mL of diethyl ether, under the condition of nitrogen protection and ice-salt bath cooling, n-butyllithium (8mL, 2.5mol / L) was added dropwise to the reaction system, and the dropwise After that, it was slowly raised to room temperature, and the reaction was stirred for 3 hours. Bis(3,5-di-tert-butylphenyl)phosphorous chloride (8.90 g, 20 mmol) was added dropwise under cooling in an ice-salt bath. After the drop was completed, the temperature was slowly raised to room temperature, and the reaction was stirred for 24 hours. The reaction was quenched with saturated sodium bicarbonate solution, extracted with dichloromethane, dried over anhydrous sodium sulfate, concentrated and column chromatographed to obtain product 7 (3.79g, 57%).
[0038]
[0039] Compound 7 (3.33 g, 5 mmol) was dissolved in 4.0 mL of acetic anhydride and reacted at 55° C. for 4 hours. After t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com