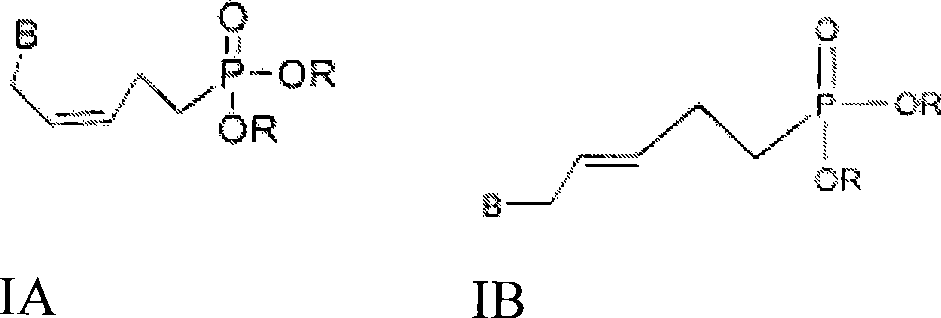
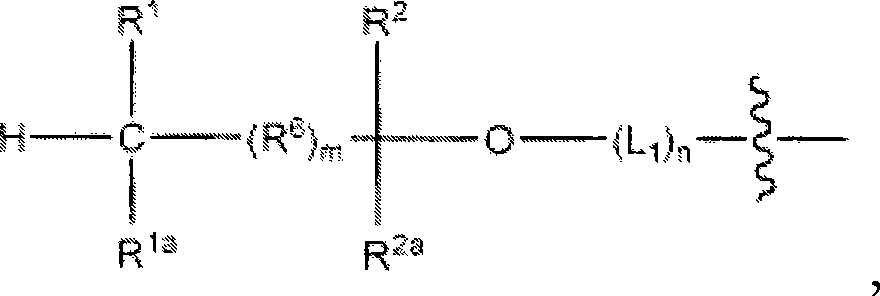
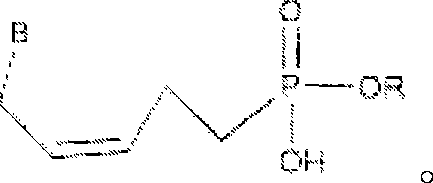Phosphono-pent-2-en-1-yl nucleosides and analogs
An acyl and alkenyl technology, used in the treatment, treatment of various diseases, or improvement of a variety of medical conditions related to viral infection and cell proliferation, preventive fields, can solve problems such as low selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0129] C. Preparation of compounds
[0130] Exemplary methods for the preparation of 5-phospho-pent-2-en-1-yl nucleosides and esters thereof for use in the compositions and methods provided herein are described below and in the Examples. Other methods known in the art can also be used to prepare the 5-phospho-pent-2-en-1-yl nucleoside and its esters provided by the present invention.
[0131] Scheme I outlines the synthesis of key intermediate 9. Example 1 provides the conditions for the synthesis of compound 9. In this procedure, 3-buten-1-ol 1 was treated with DHP and PPTS to give compound 2. Compound 2 was hydroxymethylated to give compound 3, and compound 3 was protected with TBDPSC1 to give compound 5. Compound 5 was converted to phosphate compound 7 by bromination and Arbuzov reaction. After partial hydrogenation of compound 7, the protecting group of TBDPS was removed to obtain the key intermediate 9.
[0132] Option I
[0133]
[0134] Formulations and Condit...
Embodiment 1
[0273] Synthesis of (5-hydroxy-pent-3-enyl)-diethyl phosphate
[0274] A. 2-But-3-ynyloxy-tetrahydro-pyran (2)
[0275] Dihydropyran (1.7 mL, 19 mmol) was added dropwise to CH of 3-butyn-1-ol (1.00 g, 14.3 mmol) and PPTS (0.72 g, 2.9 mmol) 2 Cl 2 solution, and the resulting mixture was stirred overnight. use CH 2 Cl 2 (80 mL) and wash the reaction mixture with 0.02N NaOH (50 mL) and brine (100 mL). The organic layer was washed with MgSO 4 Concentrated to dryness, the residue was purified by silica gel column chromatography, 3-5% EtOAc in hexane to give 1.90 g of compound 2 (12.3 mmol, 86% yield): 1 H NMR (CDCl 3 )δ4.63(t, J=3.4Hz, 1H), 3.92-3.77(m, 2H), 3.59-3.45(m, 2H), 2.47(td, J=7.1, 2.8Hz, 2H), 1.96(t , J=2.5Hz, 1H), 1.90-1.44 (m, 6H).
[0276]B. 5-(Tetrahydro-pyran-2-yloxy)-pent-2-yn-1-ol (3)
[0277] A 1.6 M solution of n-BuLi in hexane (58 mL, 92.8 mmol) was added dropwise to a solution of compound 2 (11.0 g, 71.3 mmol) in THF (80 mL) at 0°C over a period of 2...
Embodiment 2
[0291] Synthesis of 9-(5-phosphono-pent-2-en-1-yl)-adenine (13, PPen-A)
[0292] A. 9-(5-phosphono-pent-2-en-1-yl)-adenine diethylphosphonoester (10)
[0293] A solution of adenine (0.49 g, 0.36 mmol) in DMF was added to a flask containing compound 9 (0.32 g, 1.4 mmol). At 0°C, with Ph 3 The resulting mixture was treated sequentially with P (0.94 g, 0.36 mmol) and DIAD (0.70 mL, 0.36 mmol). After overnight, the mixture was concentrated and subjected to silica gel column chromatography with 5-10% MeOH in CH 2 Cl 2 Solution purification of the residue afforded 0.2 g of compound 10 (0.589 mmol, 42% yield): 1 HNMR (CDCl 3 )δ8.36(s, 1H), 7.86(s, 1IH), 5.83(brs, 2H), 5.82-5.61(m, 2H), 4.86(d, J=6.6Hz, 2H), 4.18-4.02(m , 4H), 2.63-2.49(m, 2H), 1.88(dt, J=17.9, 7.4Hz, 2H), 1.32(t, J=6.9Hz, 6H); 31 P NMR (CDCl 3 )δ31.71; MS (ESI) m / z 340 (M+H) + .
[0294] B. 9-(5-phosphono-pent-2-en-1-yl)-adenine (13, PPen-A)
[0295] A solution of compound 10 (0.300 g, 0.884 mmol) in aceto...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com