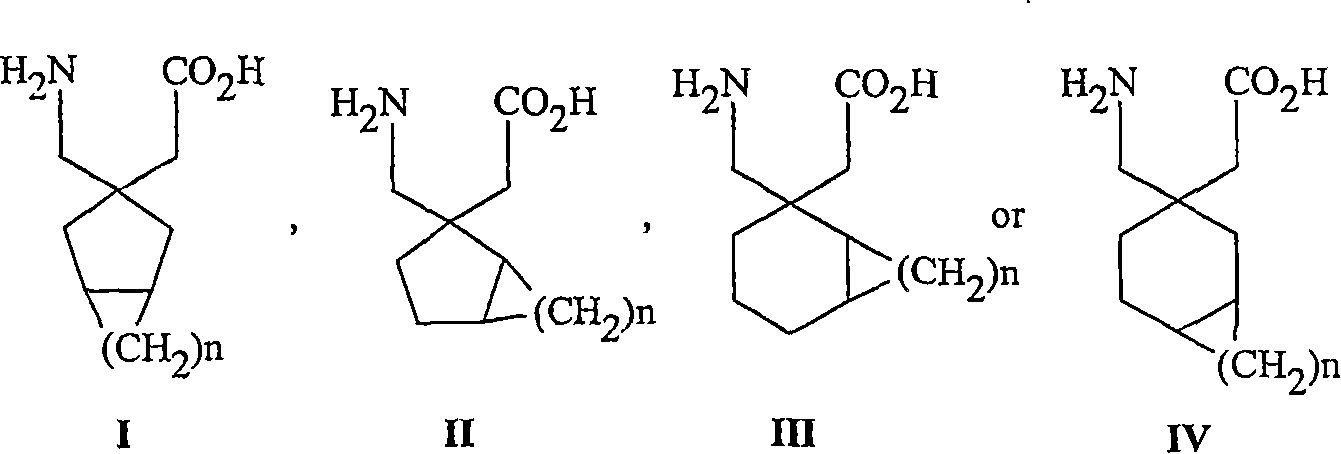
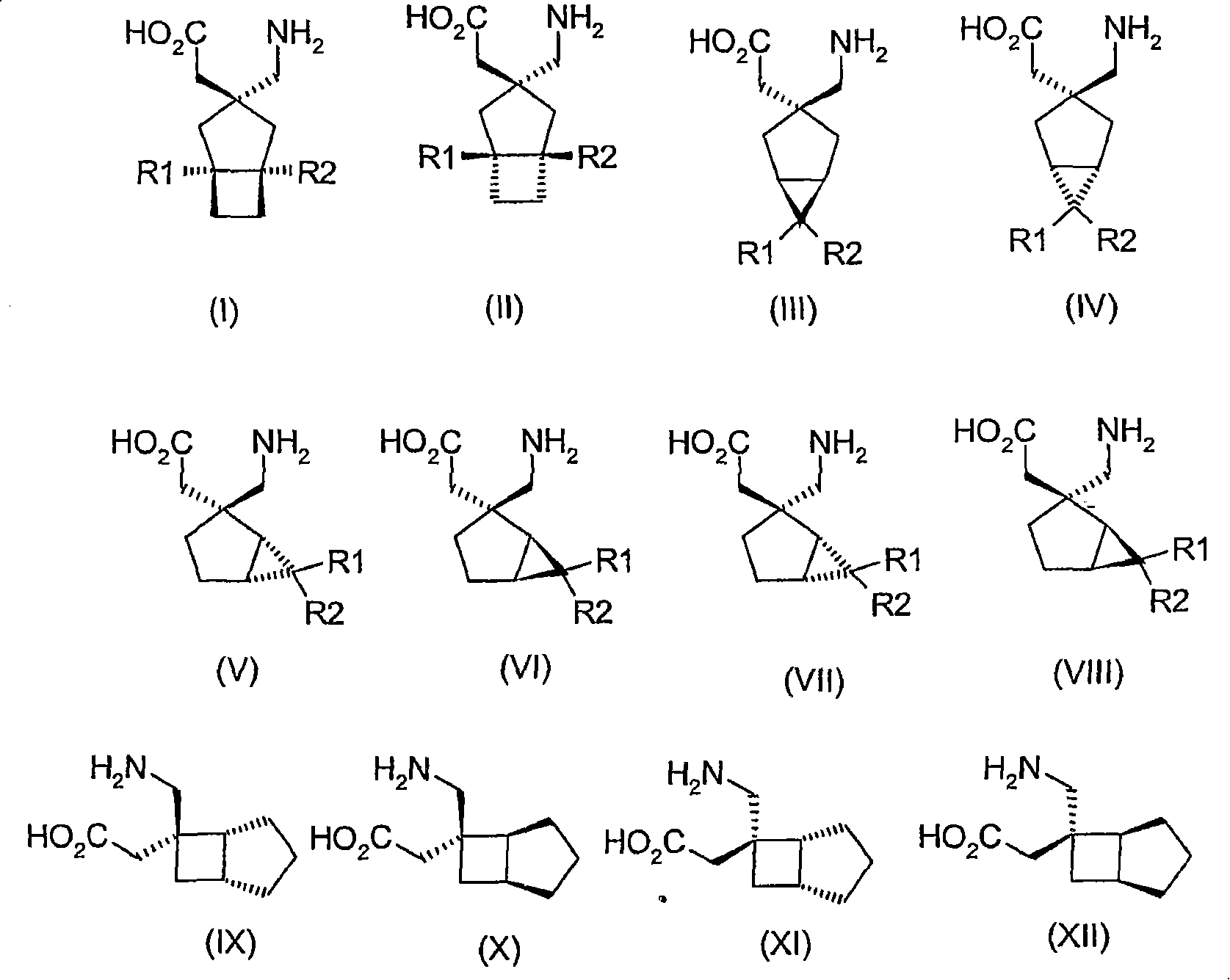
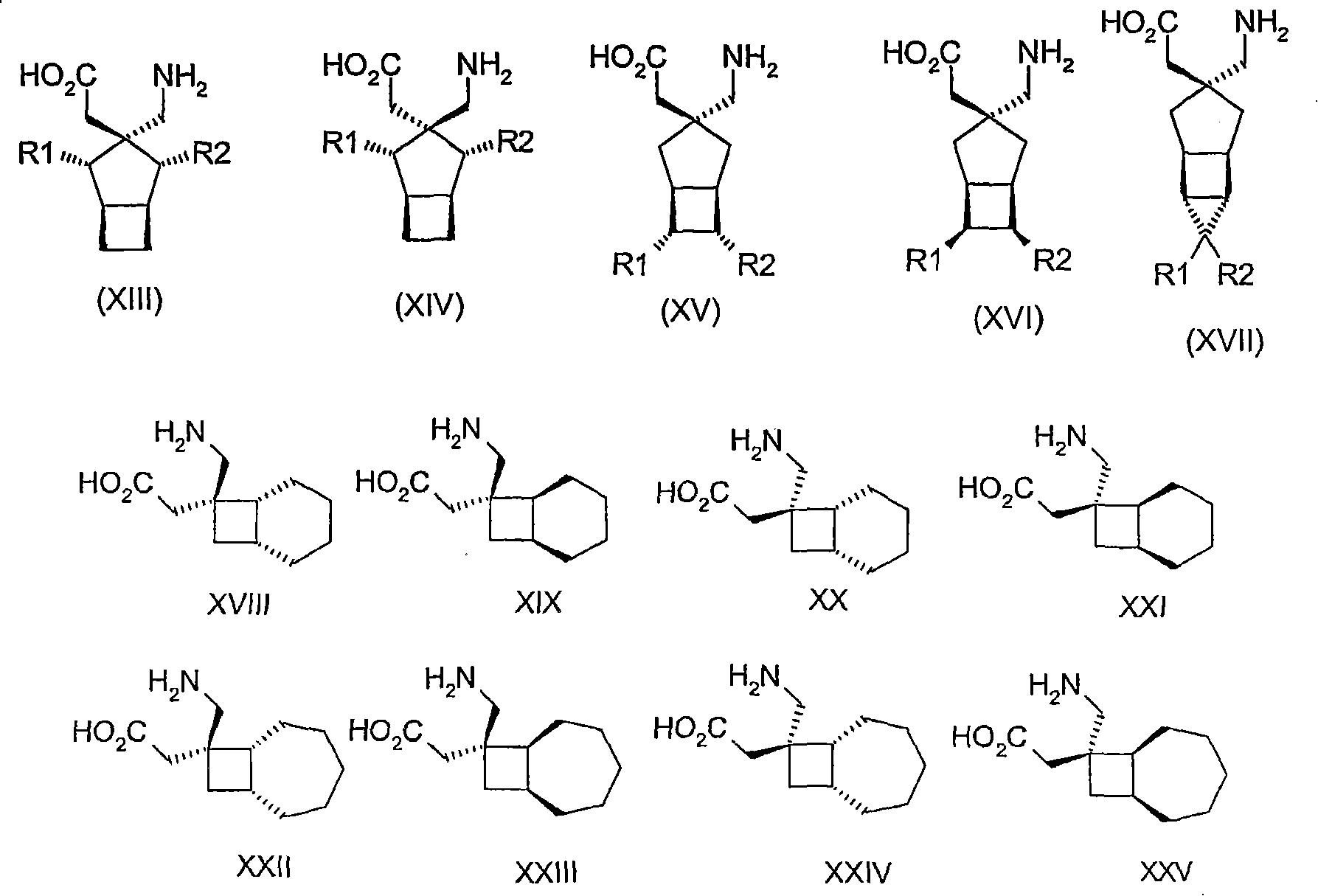
Use of reboxetine for the treatment of pain
A technology for inflammatory pain and cancer pain, applied in the application field of reboxetine in the treatment of pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0152] The following study was performed to evaluate the effect of (S,S)-reboxetine in the treatment of postherpetic neuralgia (PHN) patients unresponsive to gabapentin (GBP) treatment.
[0153] Study Population: Male or female patients aged 18 years or older who still have pain more than 3 months after the shingles rash has healed and who are also refractory to GBP treatment. There is no upper limit on the duration of PHN. GBP futility is defined as follows: Patients who show little or no improvement in Patient Global Impression of Change (PGIC) after a 2-week period of treatment with GBP (1800 mg / day) dosing, or who cannot tolerate less than or equal to 1800 mg / day Patients with GBP dosage.
[0154] Study Design: A randomized, double-blind, placebo-controlled, two-treatment (placebo and active drug) age-split study in PHN patients aged 18 years or older. The study consisted of 3 phases: (i) 7 weeks of screening, including 4 weeks of GBP treatment, (ii) 5 weeks of randomize...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com