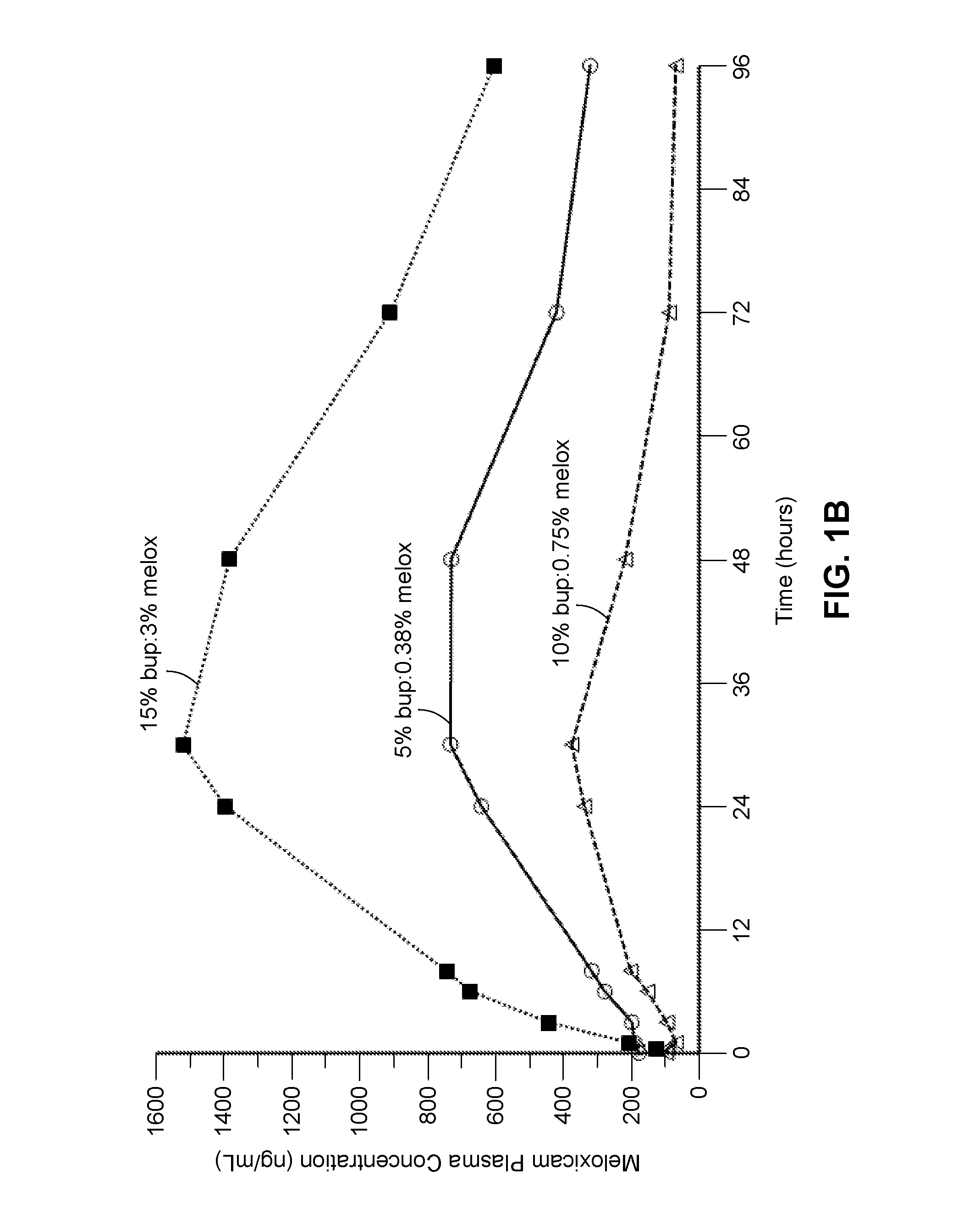
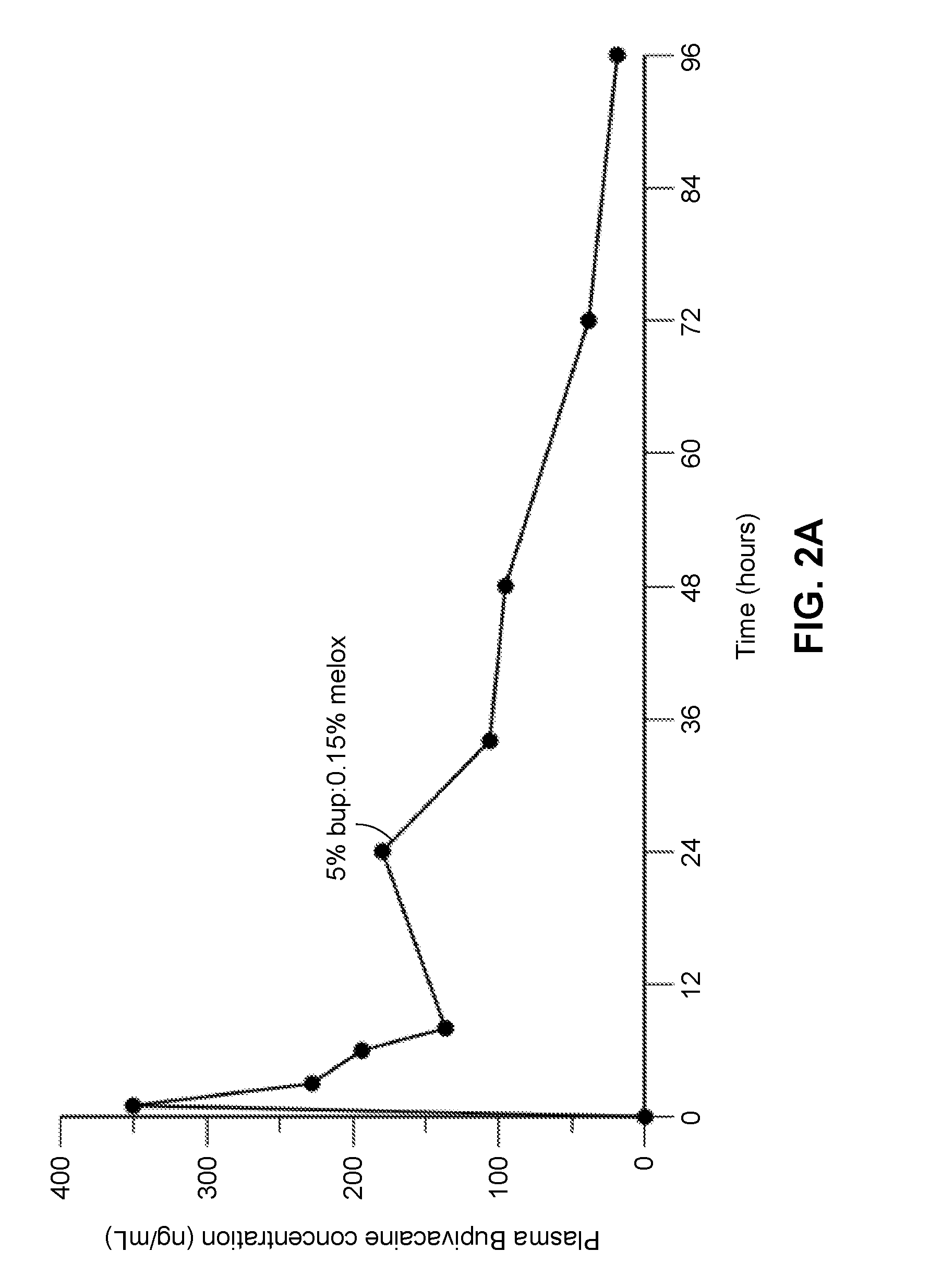
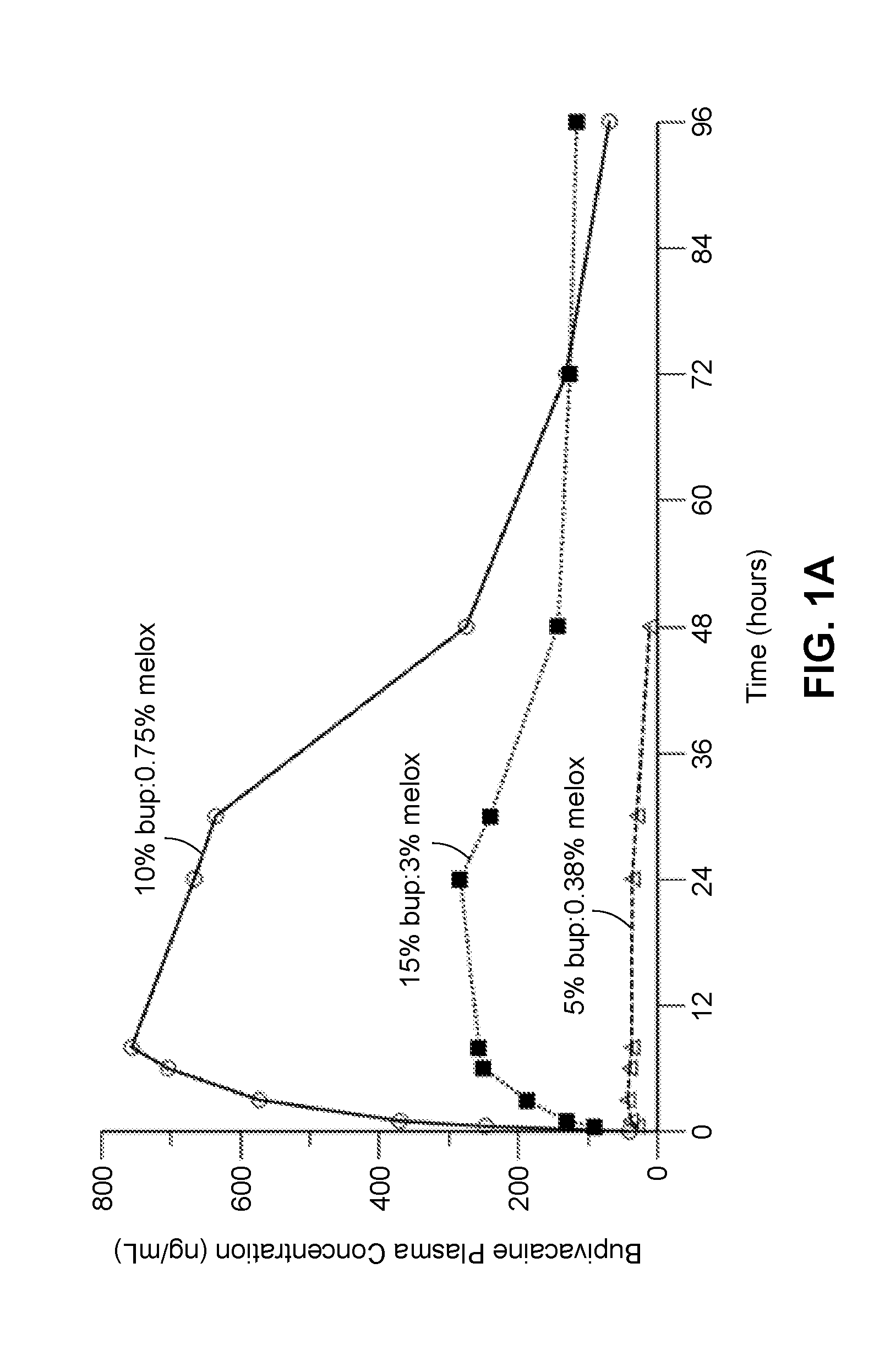
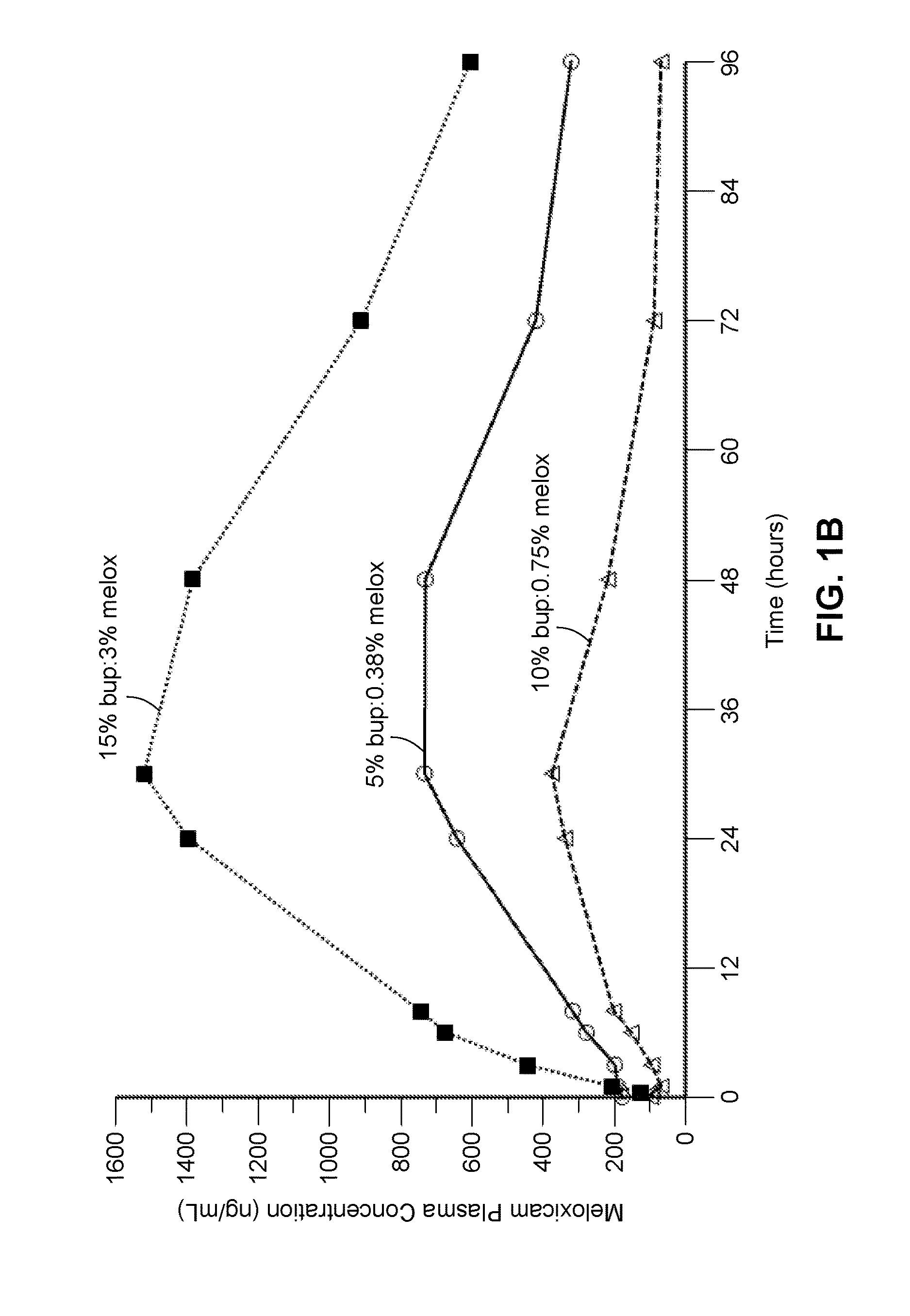
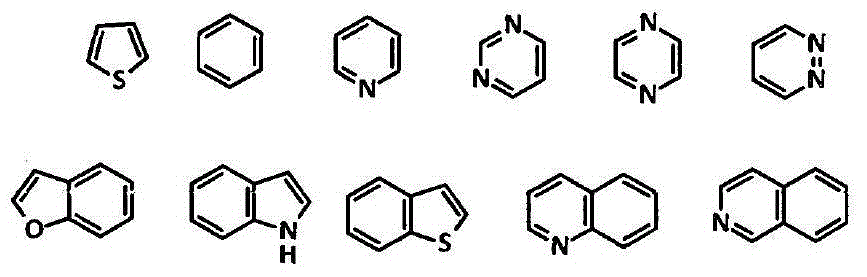
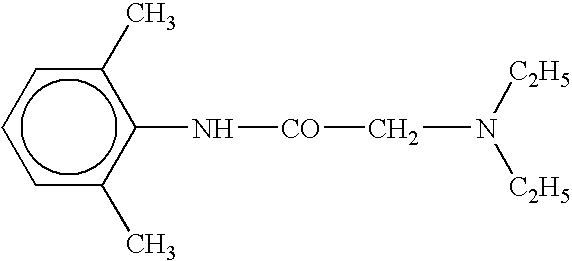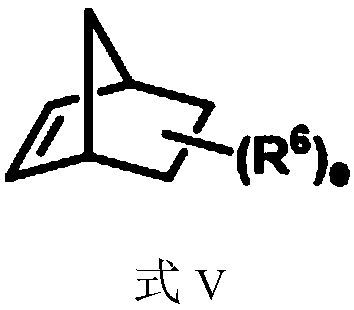Patents
Literature
63 results about "Post operative pain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
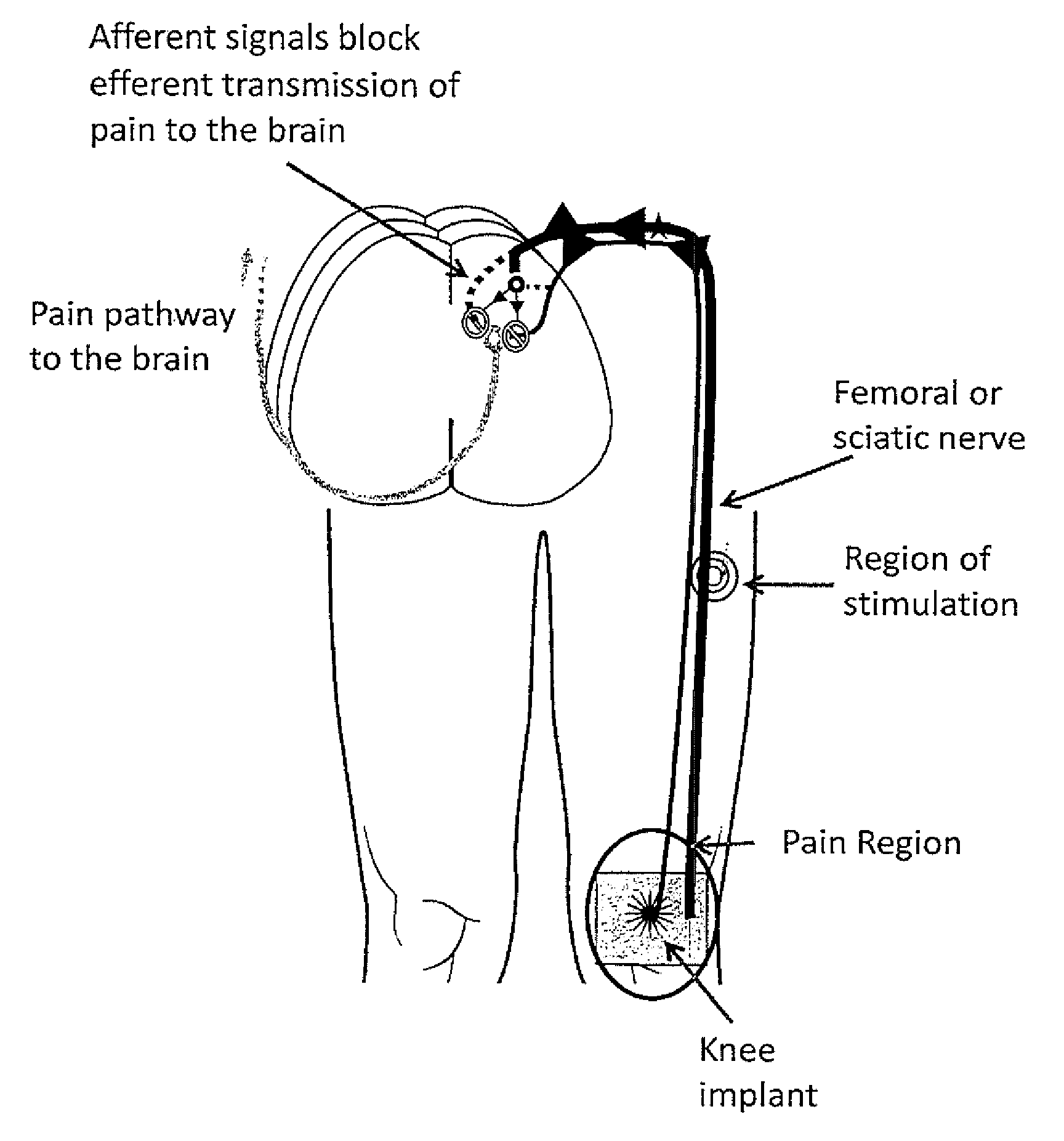
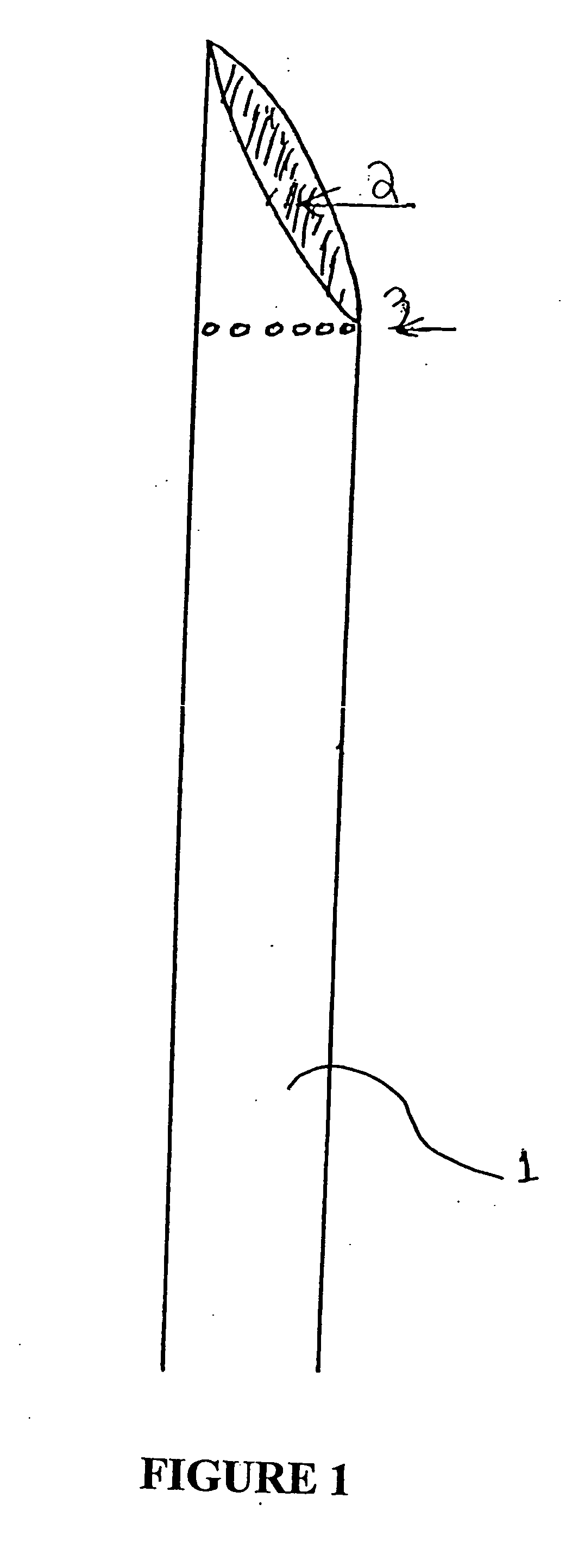
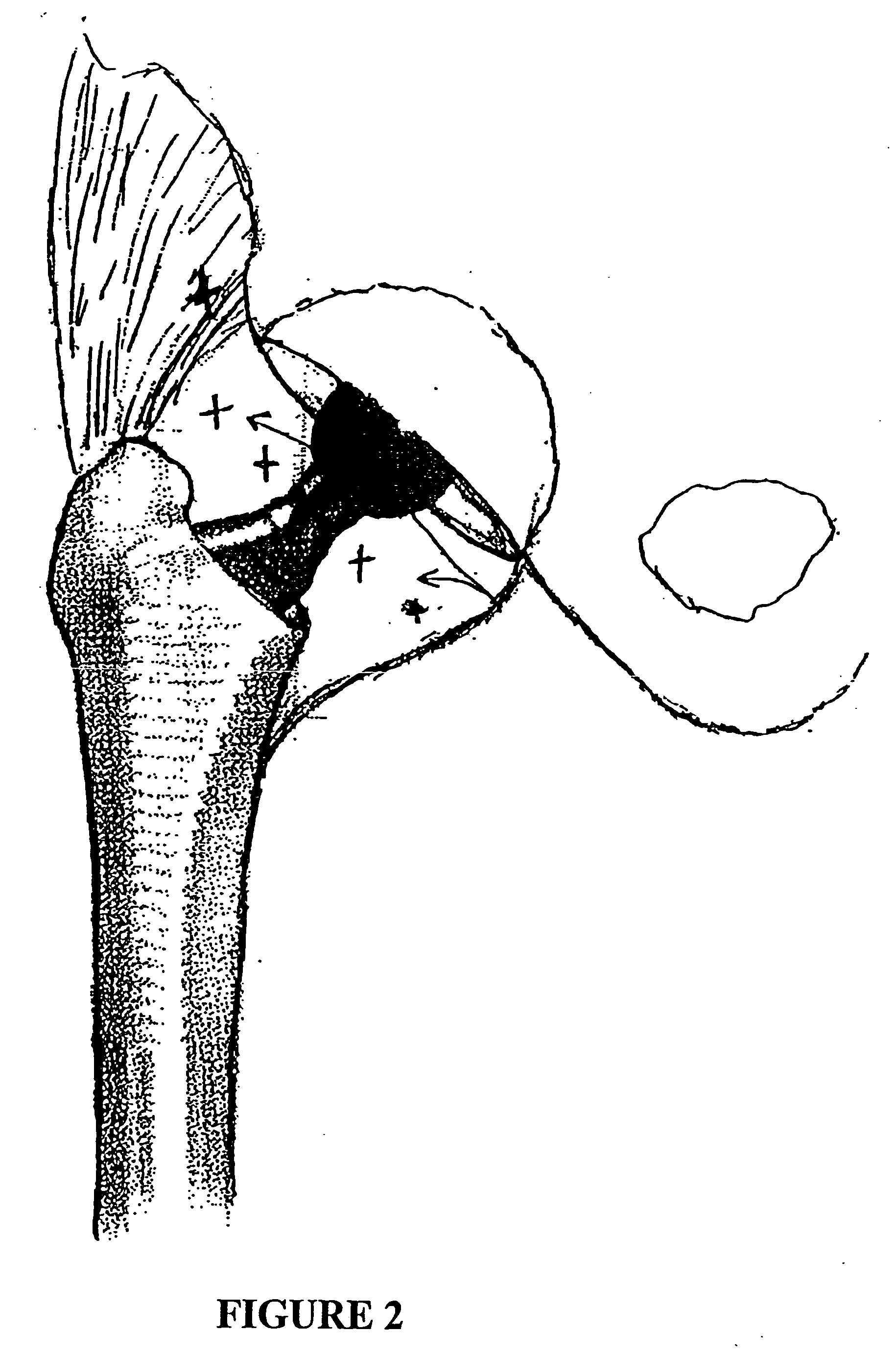
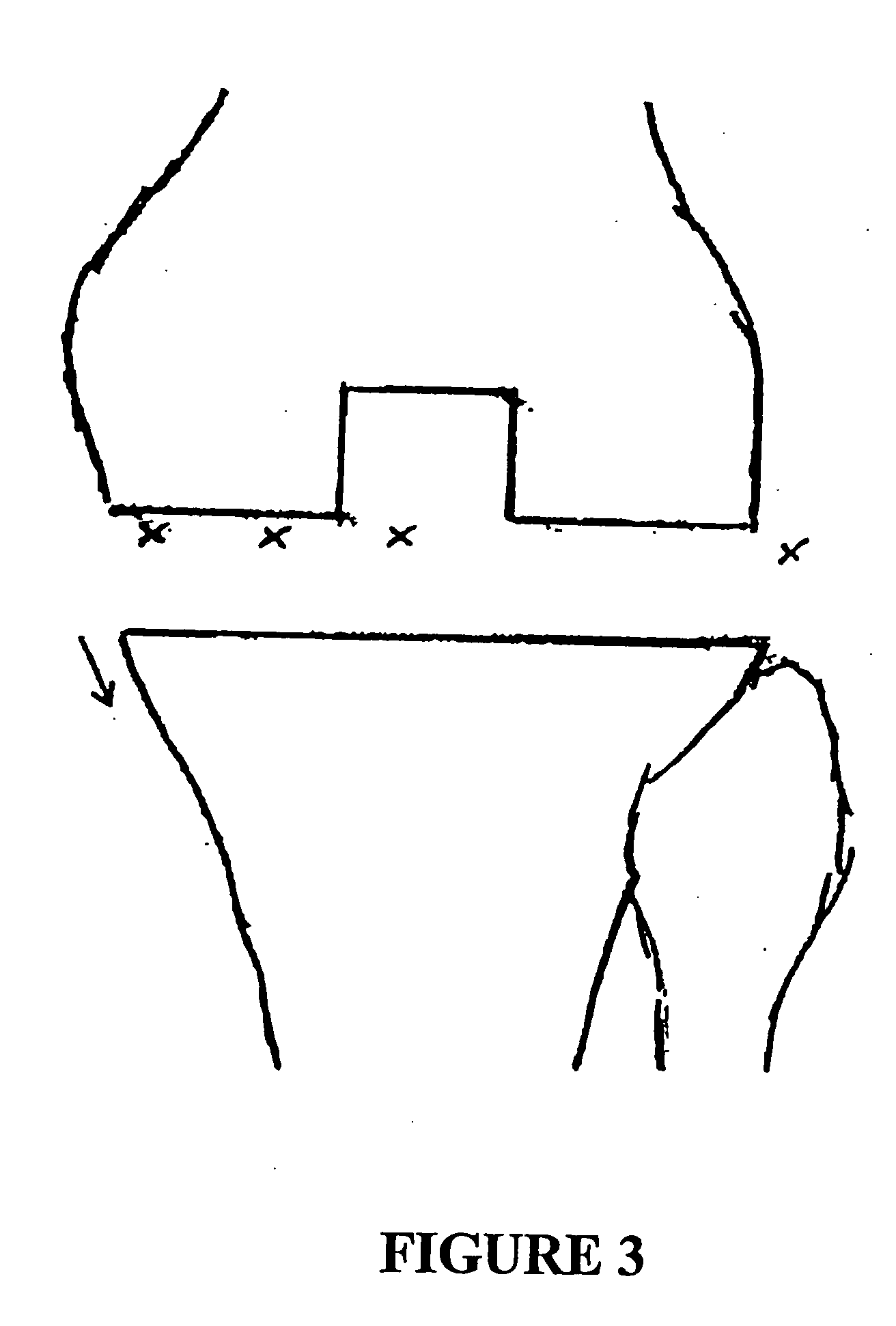
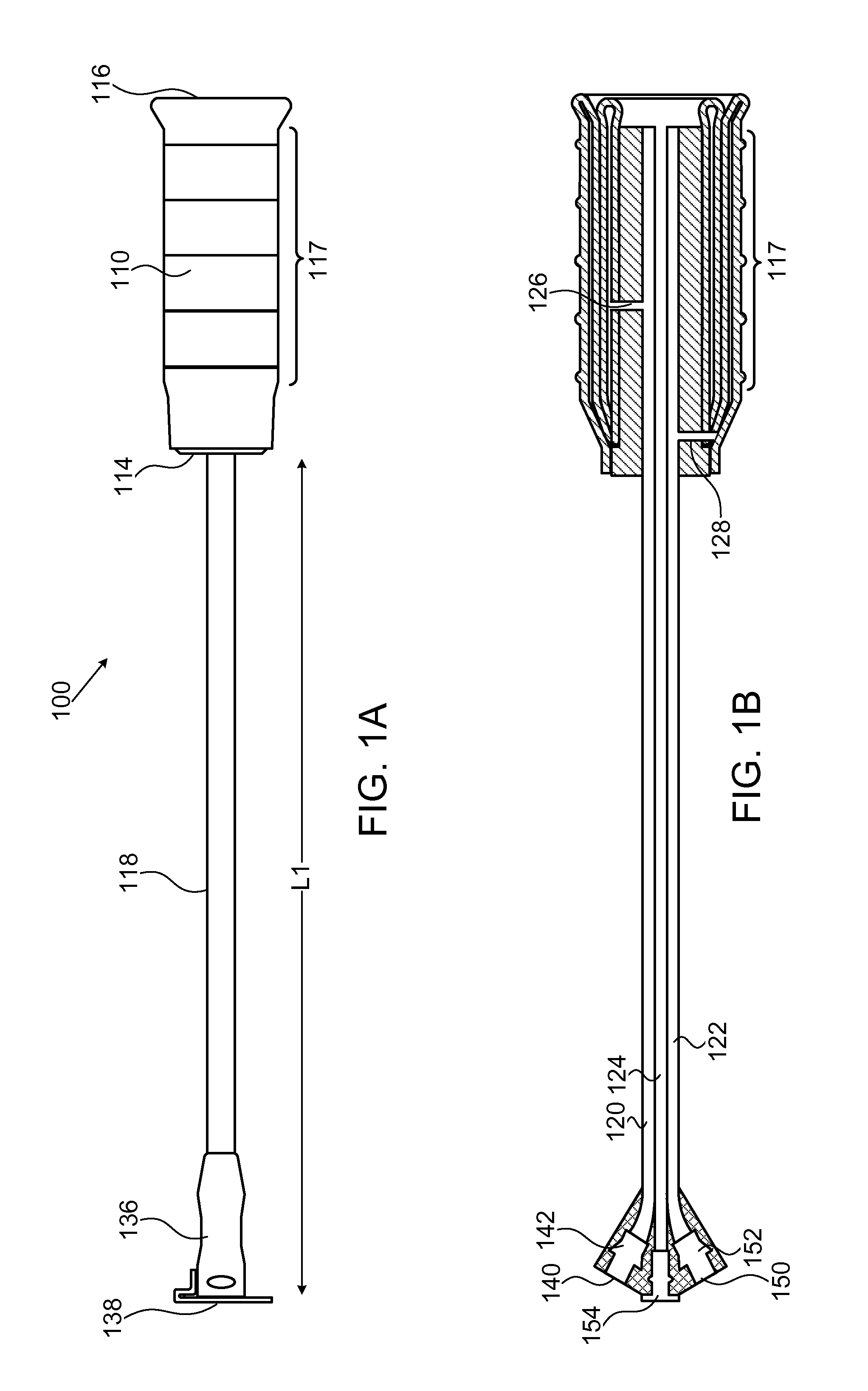
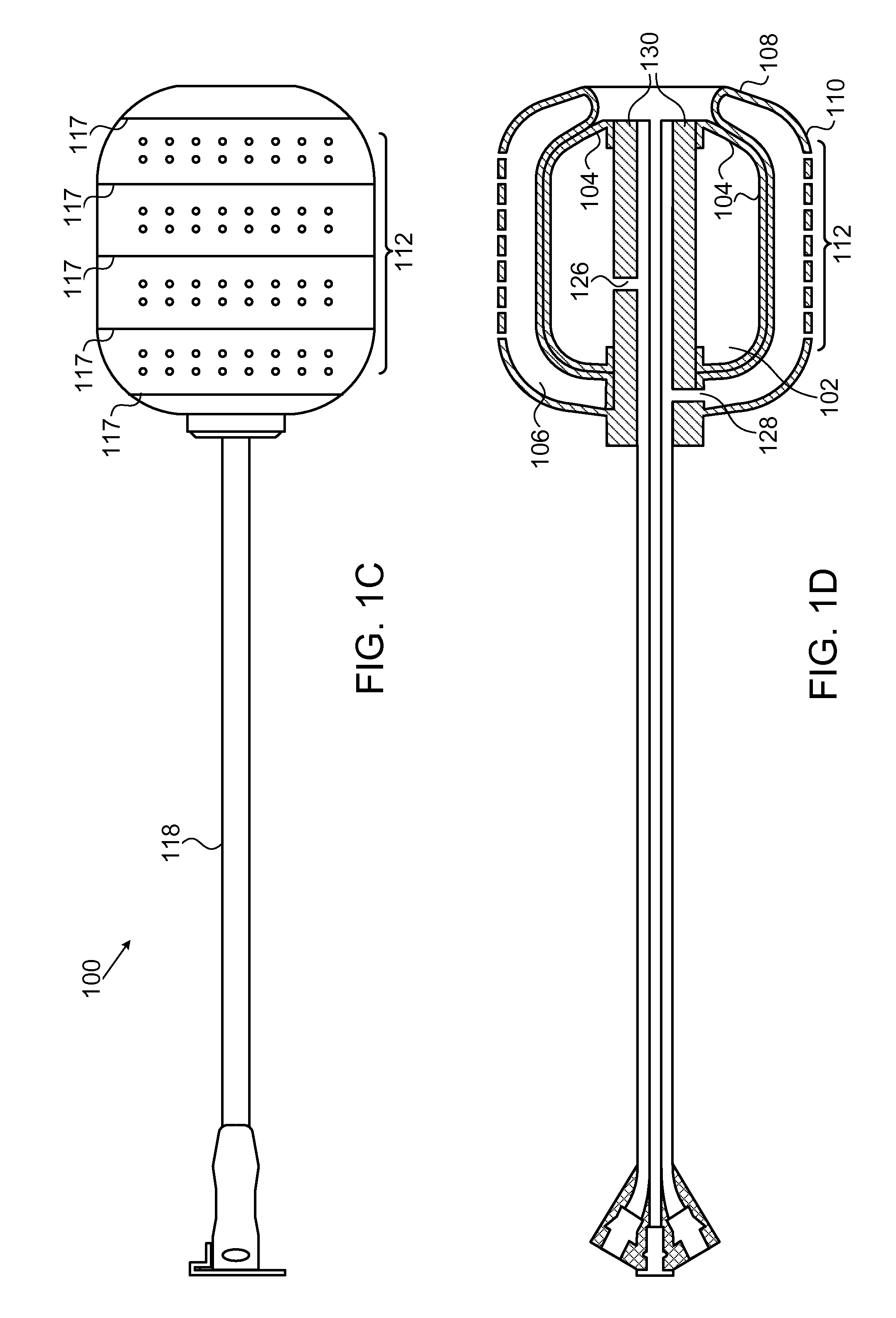
System and method for treatment of pain related to limb joint replacement surgery
It has been discovered that pain felt in a given region of the body can be treated by stimulating a peripheral nerve at a therapeutically effective distance from the region where pain is felt to generate a comfortable sensation (i.e., paresthesia) overlapping the regions of pain. A method has been developed to reduce pain in a painful region following limb joint replacement by stimulating a peripheral nerve innervating the painful region with an electrode inserted into tissue and spaced from the peripheral nerve. This method may be used to help alleviate postoperative pain in patients following total knee arthroplasty surgery or other limb joint replacement surgeries.
Owner:SPR THERAPEUTICS
Device and method for endovascular treatment for causing closure of a blood vessel
ActiveUS20080188843A1Reduce power densityReduce temperature peaksCatheterSurgical instrument detailsEndovascular treatmentLaser light
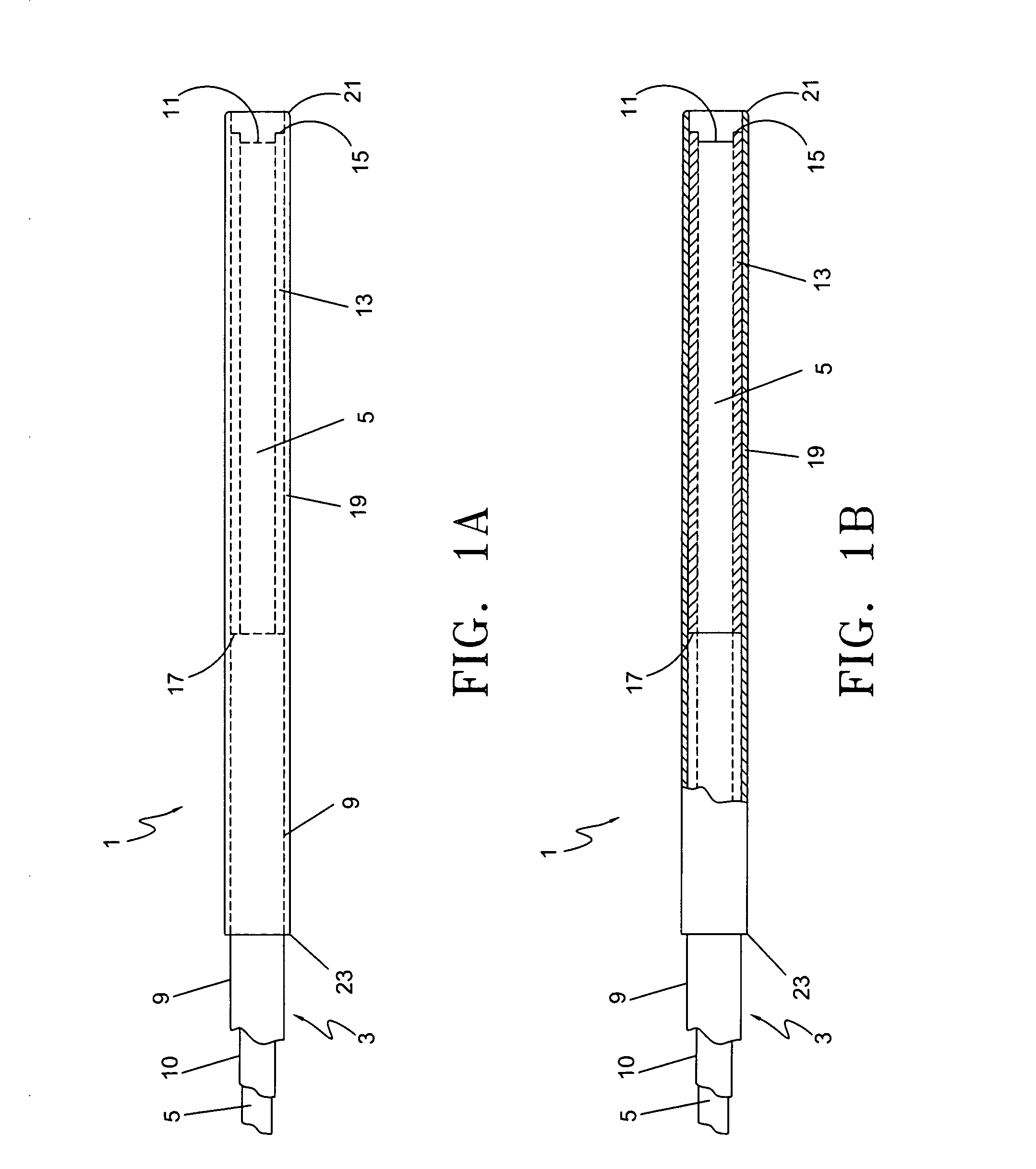
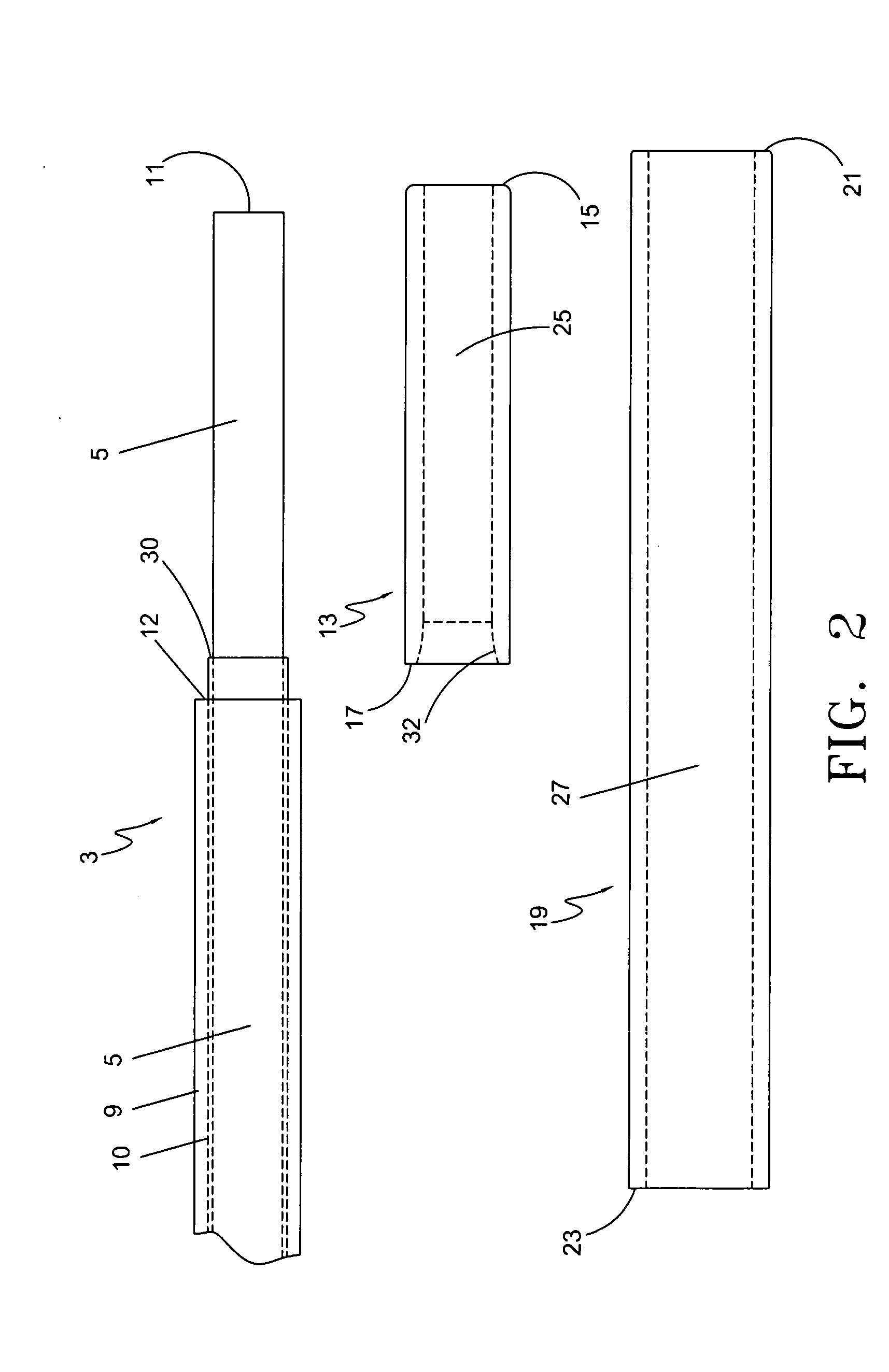
An endovascular laser treatment device for causing closure of a blood vessel uses an optical fiber adapted to be inserted into a blood vessel. An inner sleeve is arranged around a distal portion of the optical fiber core such that both distal ends of the inner sleeve and the optical fiber core form an enlarged light emitting face. The enlarged emitting face provides substantially lower power density while providing the same amount of total energy during a treatment session. An outer sleeve arranged around the inner sleeve acts as a spacer to position the light emitting face away from an inner wall of the blood vessel. The enlarged light emitting face and the outer sleeve acting as a spacer reduces the possibility of thermal run-away and device damage, and reduce the possibility of vessel perforations, leading to less bruising, post-operative pain and other clinical complications. In yet another aspect of the present invention, a spacer comprises an inner sleeve and an outer sleeve both arranged around a distal portion of the core to prevent the laser light from traveling laterally and to position the light emitting face away from an inner wall of the vessel. The inner sleeve can be a heat resistive material such as ceramic and the outer sleeve can be, for example, a metallic sleeve to provide structural integrity and strength to the distal section of the treatment device.
Owner:ANGIODYNAMICS INC
Flexible Wire Transection of the Transverse Carpal Ligament
InactiveUS20060271080A1Easy to passIncrease the cross-sectional areaSurgical needlesBlunt dissectorsCross cutCarpal ligament
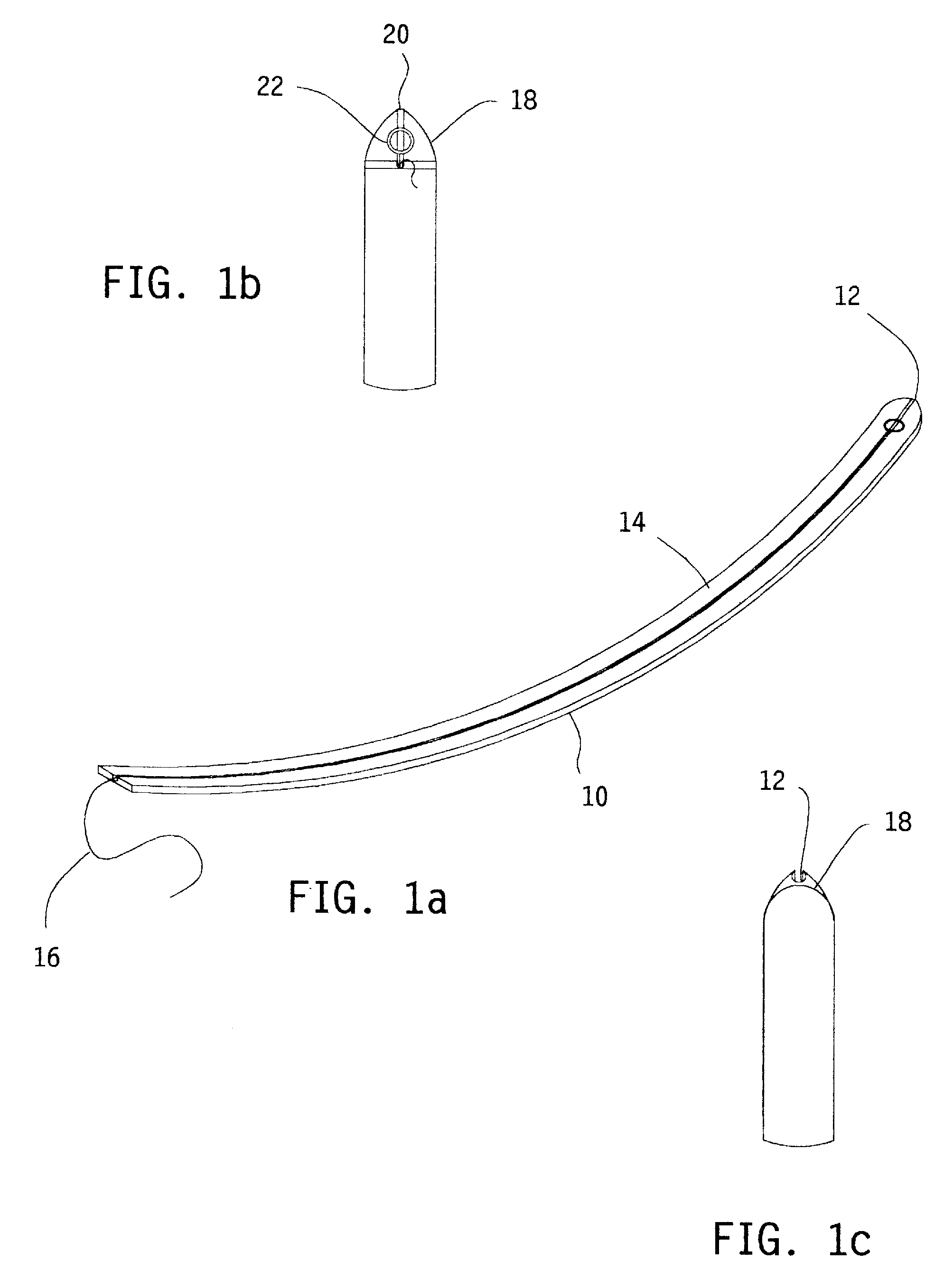
A flexible cutting filament or wire is led through the carpal tunnel, beneath the transverse carpal ligament, by a passer. Thereafter, the opposite ends of the wire are secured in an instrument which tightens the wire and may be used to move the wire as a cutting tool to transect the overlying ligament, while preserving surrounding tissues. The small wire diameter enables minimally invasive techniques to limit post-operative pain and speed recovery.
Owner:SUDDABY LOUBERT
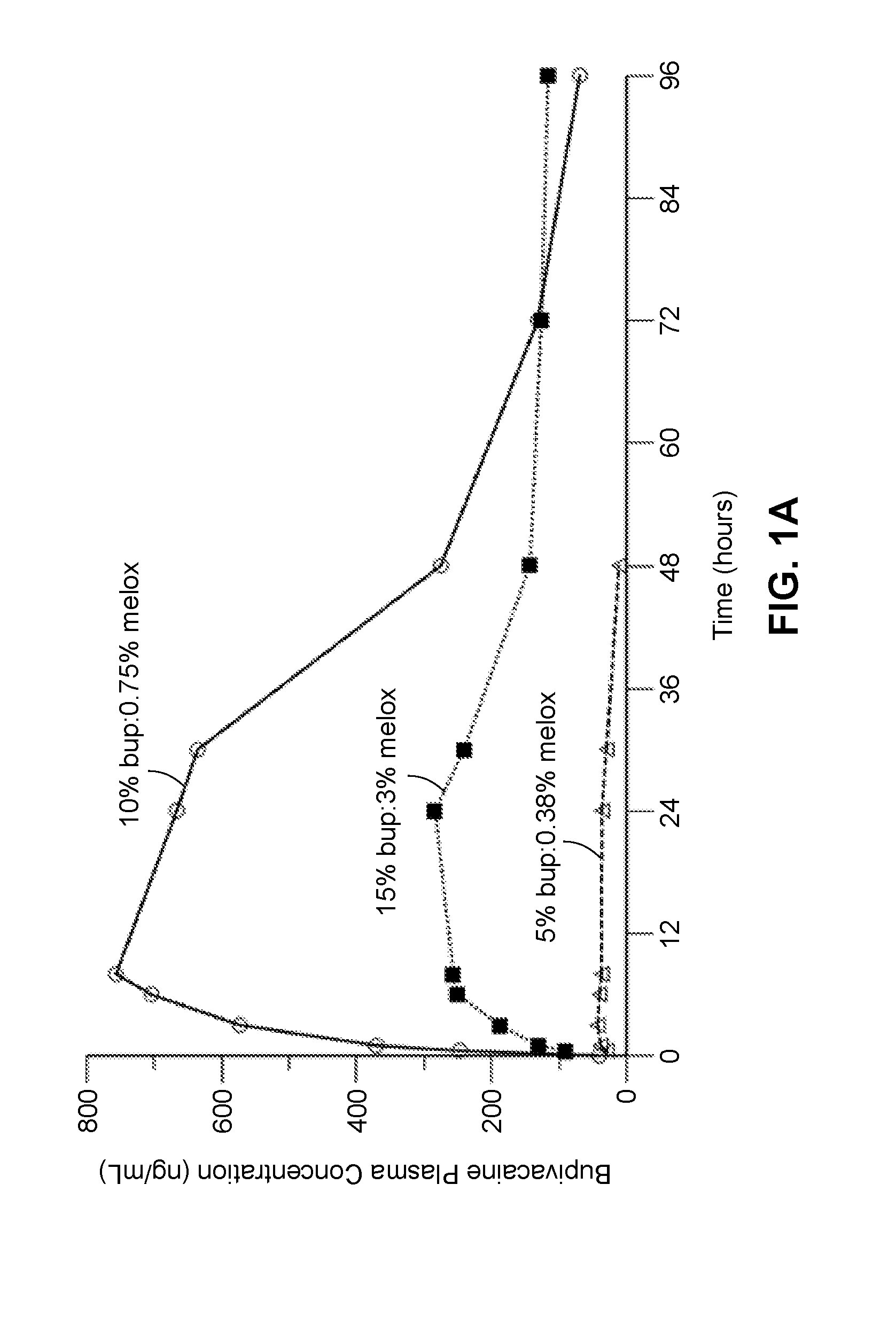
Long-acting polymeric delivery systems
ActiveUS20150297729A1Efficient releaseImprove the level ofPowder deliveryBiocideAnesthetic AgentActive agent
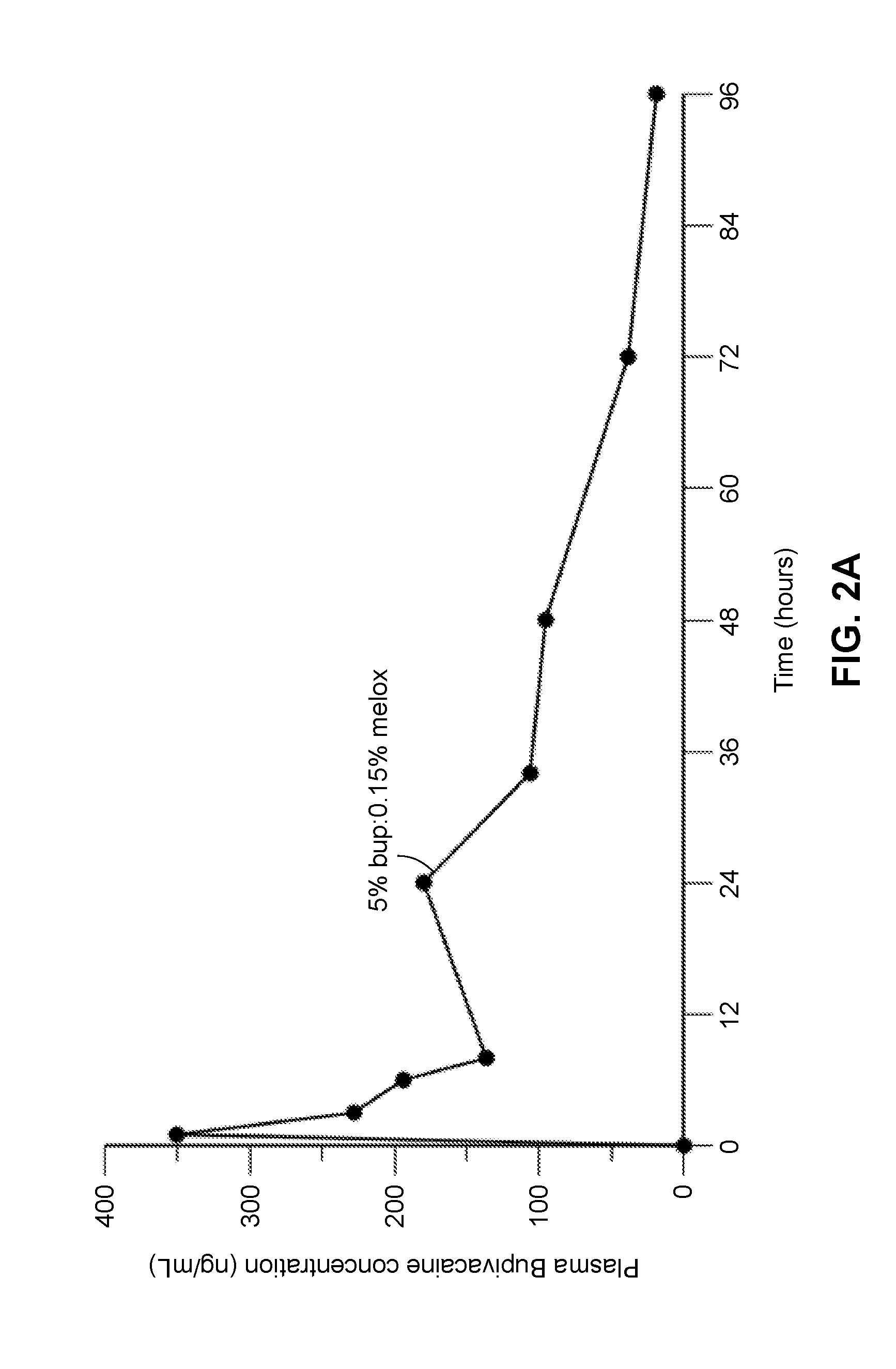
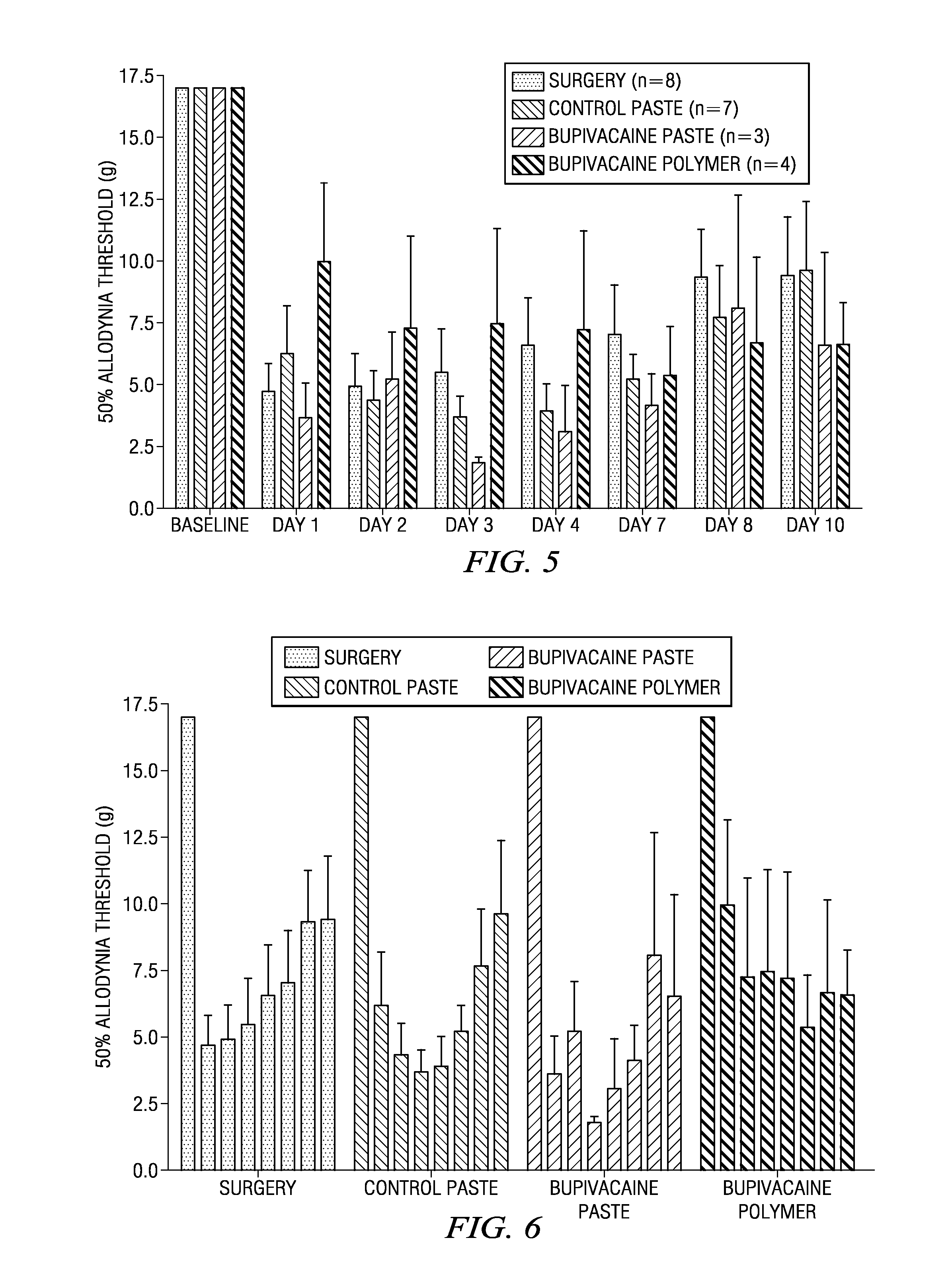
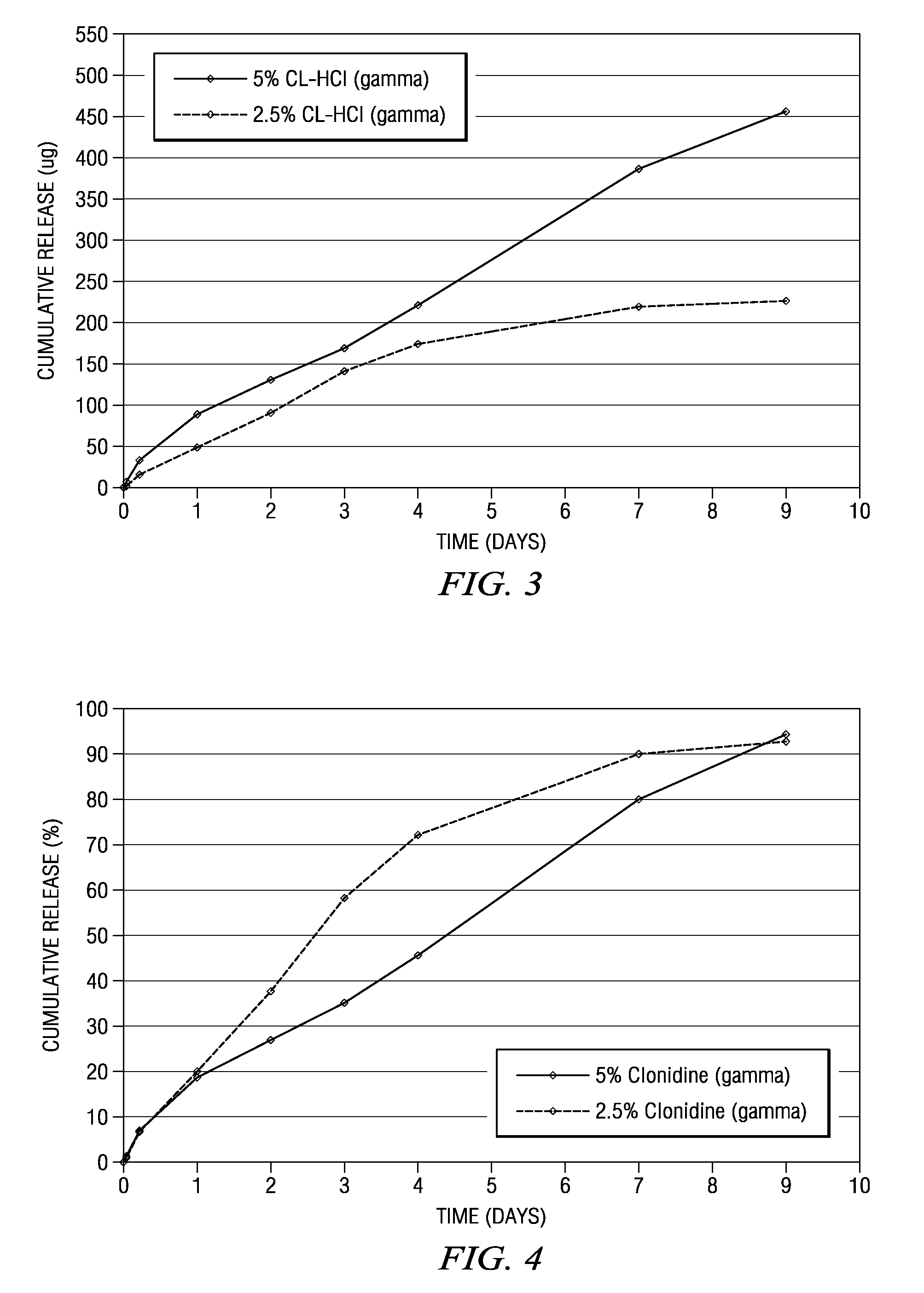
Compositions comprised of a delivery vehicle or delivery system and an active agent dispersed within the delivery vehicle or system, wherein the delivery vehicle or system contains a polyorthoester polymer and a polar aprotic solvent. Also disclosed are low viscosity delivery systems for administration of active agents. The low viscosity delivery systems have a polyorthoester polymer, a polar aprotic solvent and a solvent containing a triglyceride viscosity reducing agent. Compositions described include an amide- or anilide-type local anesthetic of the “caine” classification, and a non-steroidal anti-inflammatory drug (NSAID), along with related methods, e.g., for treatment of post-operative pain or for prophylactic treatment of pain. The compositions are suitable for delivery via, e.g., direct application and instillation, intradermal injection, subcutaneous injection, and nerve block (perineural).
Owner:HERON THERAPEUTICS
Intra-operative procedure for post-operative pain control
InactiveUS20050176823A1Eliminating post-operative painFree from painBiocideInorganic active ingredientsPost-Procedural PainSolution flow
The instant invention is directed towards an intra-operative method and kit for essentially eliminating pain associated with and resulting from surgical procedures, comprising the administration of a medical solution including a mixture of an injectable anesthetic, epinephrine, sodium chloride and an injectable anti-inflammatory agent to a plurality of sites within a surgical field utilizing a means for administration which, in a contemplated embodiment, may include a hollow shaft having a proximal end and a distal end, wherein upon administration, said medical solution flows within said shaft from said proximal end toward said distal end, which distal end is defined by a solid end having a plurality of circumferentially positioned apertures in said shaft for providing radially directed flow of the medicated solution circumferentially about said shaft.
Owner:DIAZ ROBERT L
Method for treating non-neuropathic pain
InactiveUS20060147510A1Relief the painEasy transferOrganic active ingredientsNervous disorderTransdermal patchRepetitive motion injury
A method including topically administering an effective amount of local anesthetic to a patient is disclosed. The method is effective for inducing analgesia for treating non-neuropathic pain. Non-neuropathic pain suitable for treatment according to the invention includes pain associated with sports injuries; sprains; strains; soft-tissue injury; repetitive motion injury; carpal tunnel syndrome; injury to tendons, ligament, and muscles; conditions such as fibromyalgia, bursitis, castrochondritis, myofascial pain, and pain associated with arthritis, inflammation, contusions, post-surgical pain, and nociceptive pain. Preferably, the lidocaine is applied via a transdermal patch applied near the locus of pain.
Owner:ENDO PHARMA INC
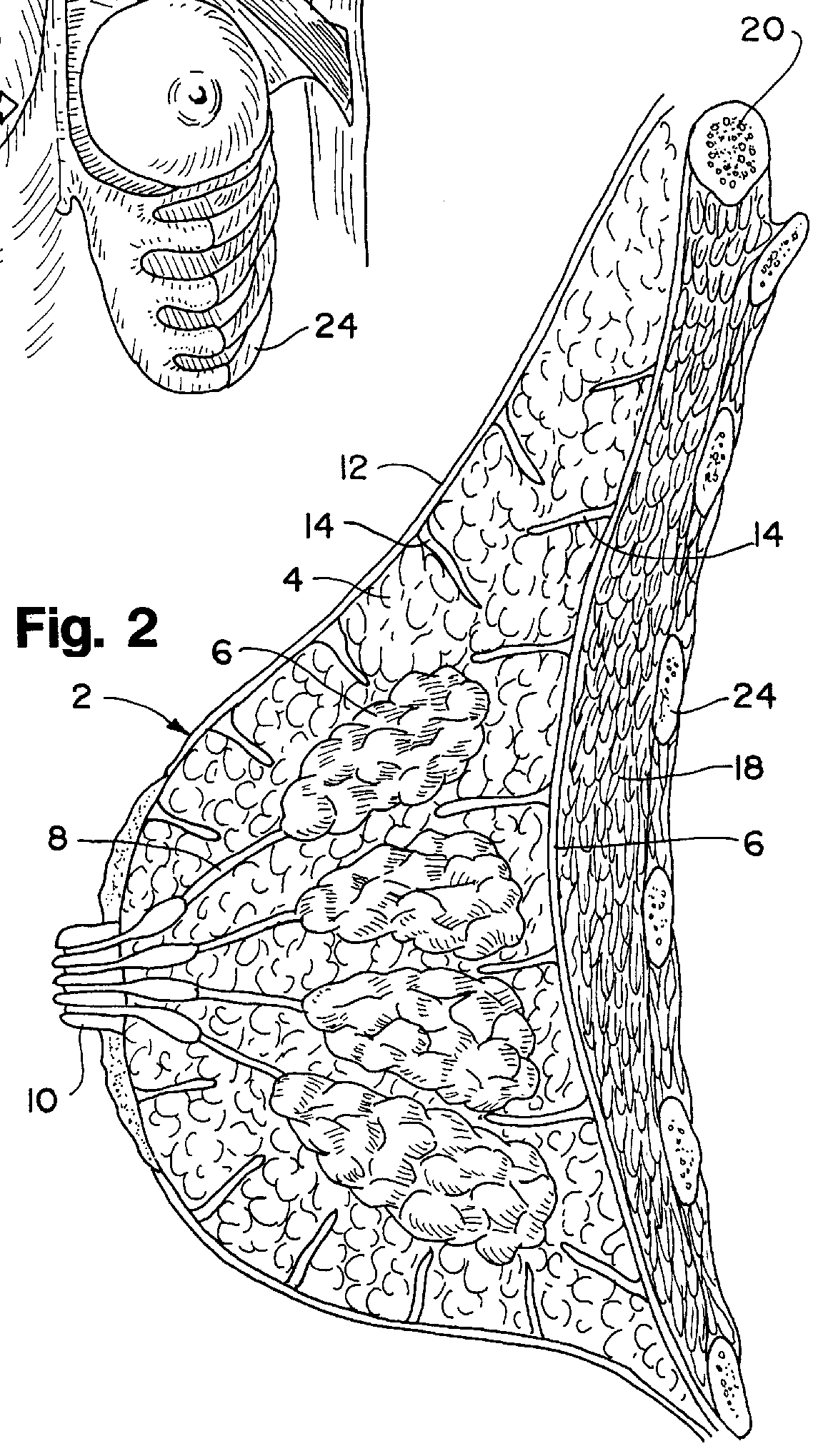
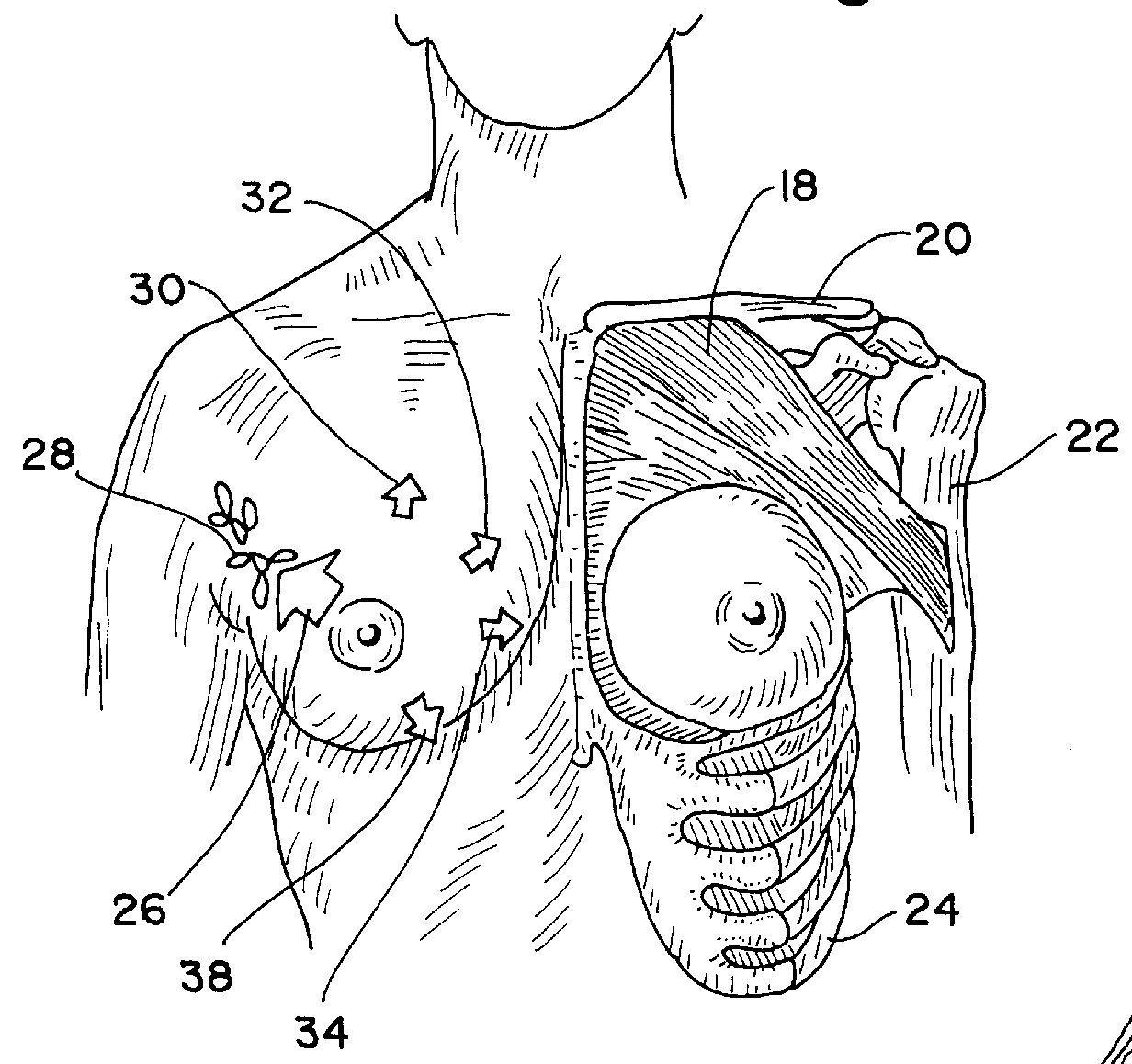
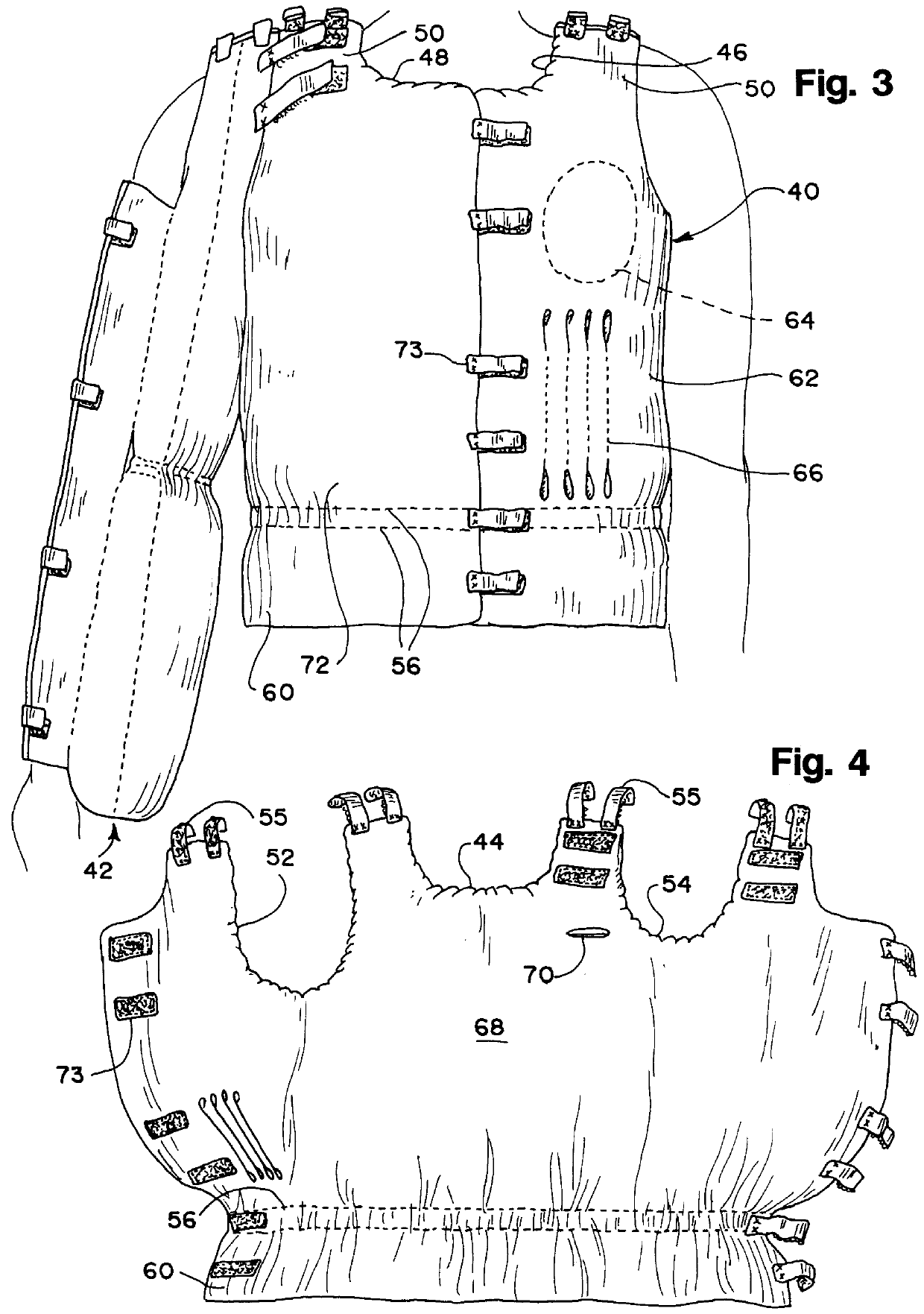
Post-mastectomy garment
InactiveUSRE36869E1Reduce postoperative painEasy to optimizeJacketsBrassieresRecovery periodPost operative pain
A garment for use by a patient after surgery for breast removal which alleviates post-operative pain and discomfort and facilitates normal activities during the recovery period. A padded vest-like garment is adapted for applying comforting pressure to the sites of removal of breast and other tissues and for holding pain relieving packages. A detachable arm support provides further comfort in a similar manner.
Owner:EWEN CAROL J
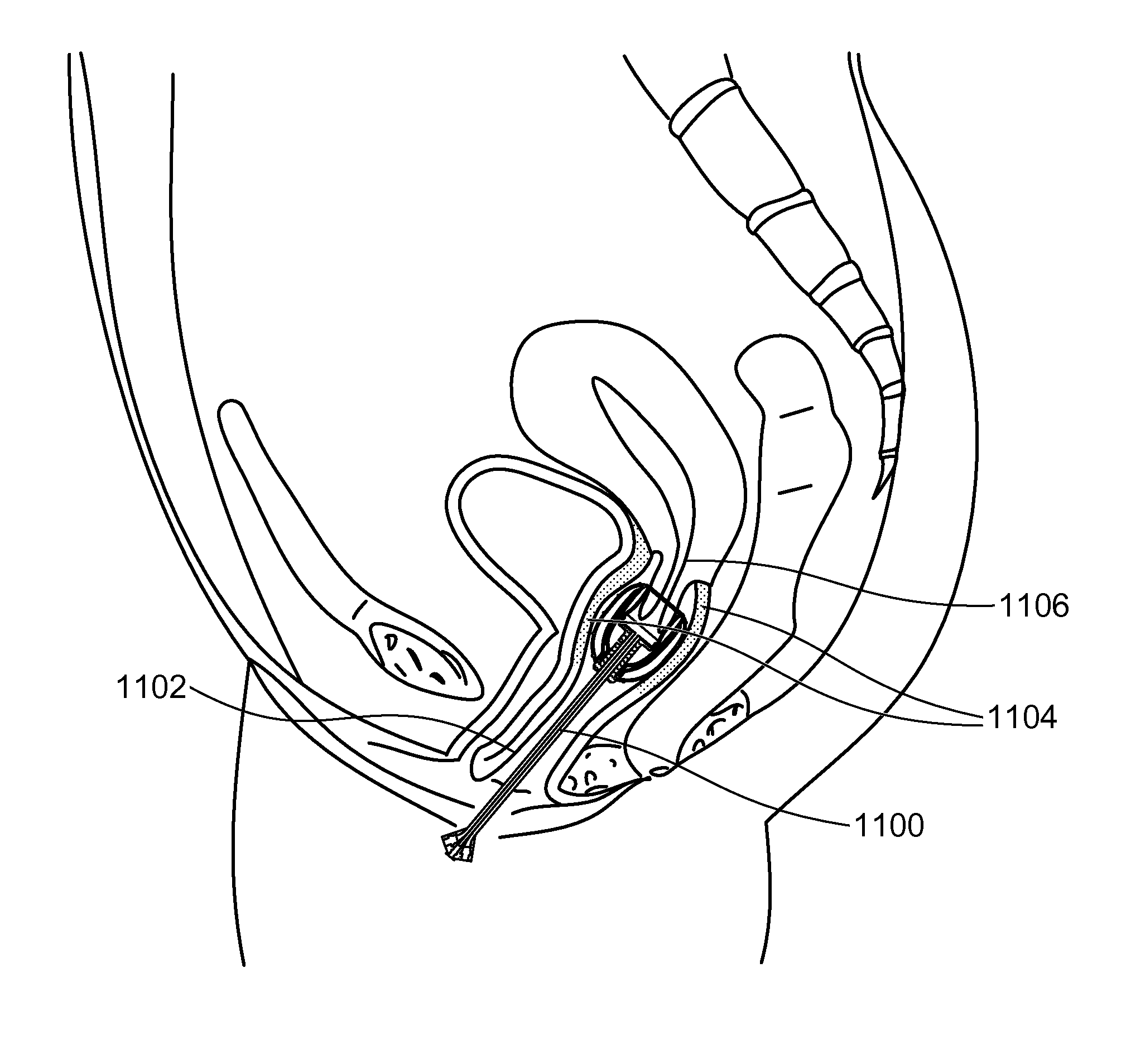
Stent devices for support, controlled drug delivery and pain management after vaginal surgery
InactiveUS20130138134A1Good supportControl drug deliveryStentsBalloon catheterVaginal epitheliumControl drugs
Stent devices comprising an inflatable inner balloon defined by an envelope and an outer balloon surrounding the inner balloon concentrically, the outer balloon serving as a therapeutic agent reservoir used for controlled therapeutic agent delivery. In some embodiments, the outer balloon has two separate walls which define its volume and is independently inflatable. In some embodiments, the two concentric balloons form a substantially cylindrical structure which has a proximal end and a distal end. A catheter carrying inflation and drainage lumens extends outward from the proximal end. In some embodiments, the distal end includes an embedded cervix accommodating tip or has a non-embedded cervix accommodating tip attached thereto. The stent devices can be used to maintain the integrity and placement of vaginally placed mesh or graft after reconstructive procedures, prevent vaginal hematoma formation and bleeding, provide pain control after surgery and deliver antibiotics or hormones to the vaginal epithelium.
Owner:ELMAN NOEL M +1
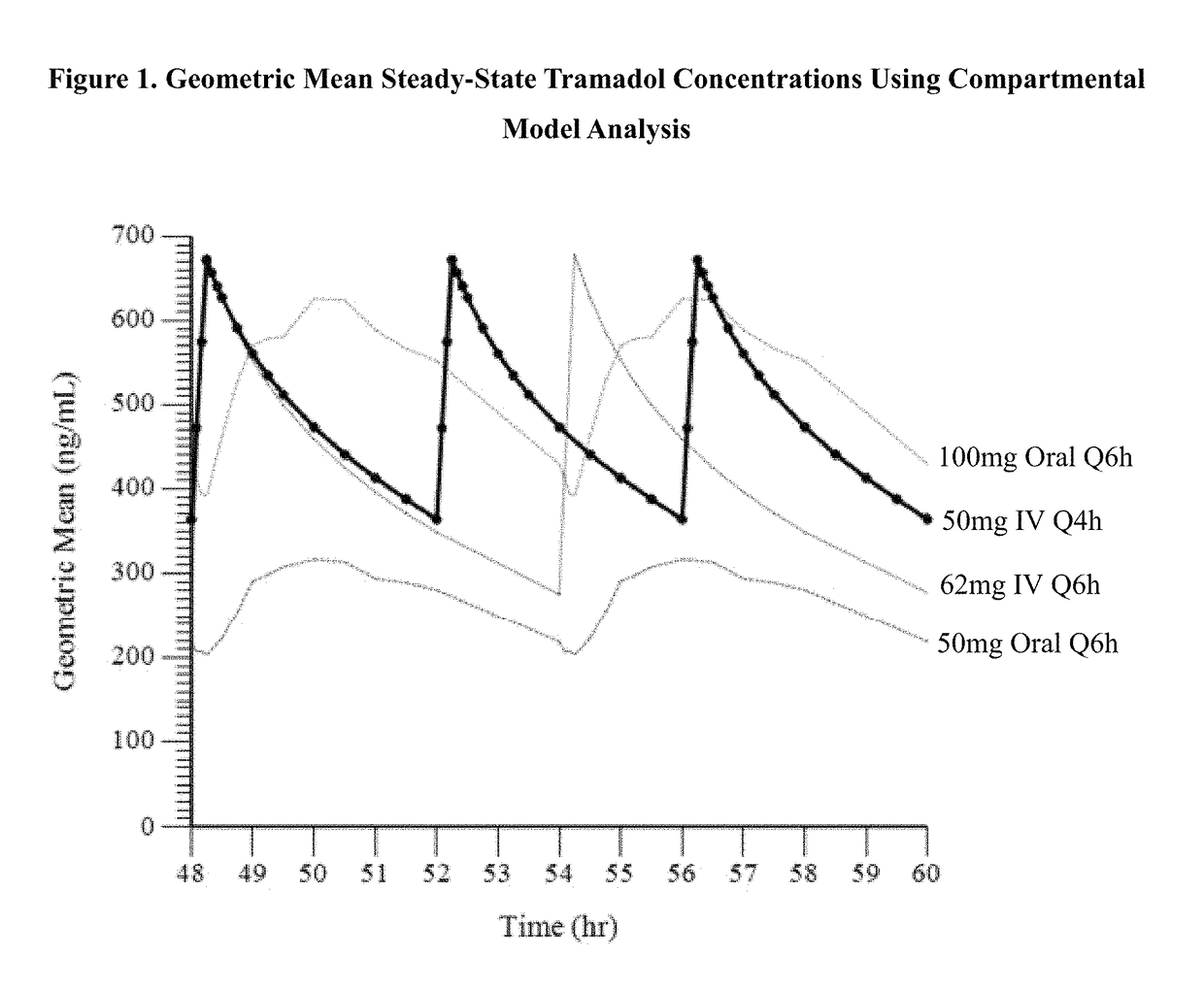
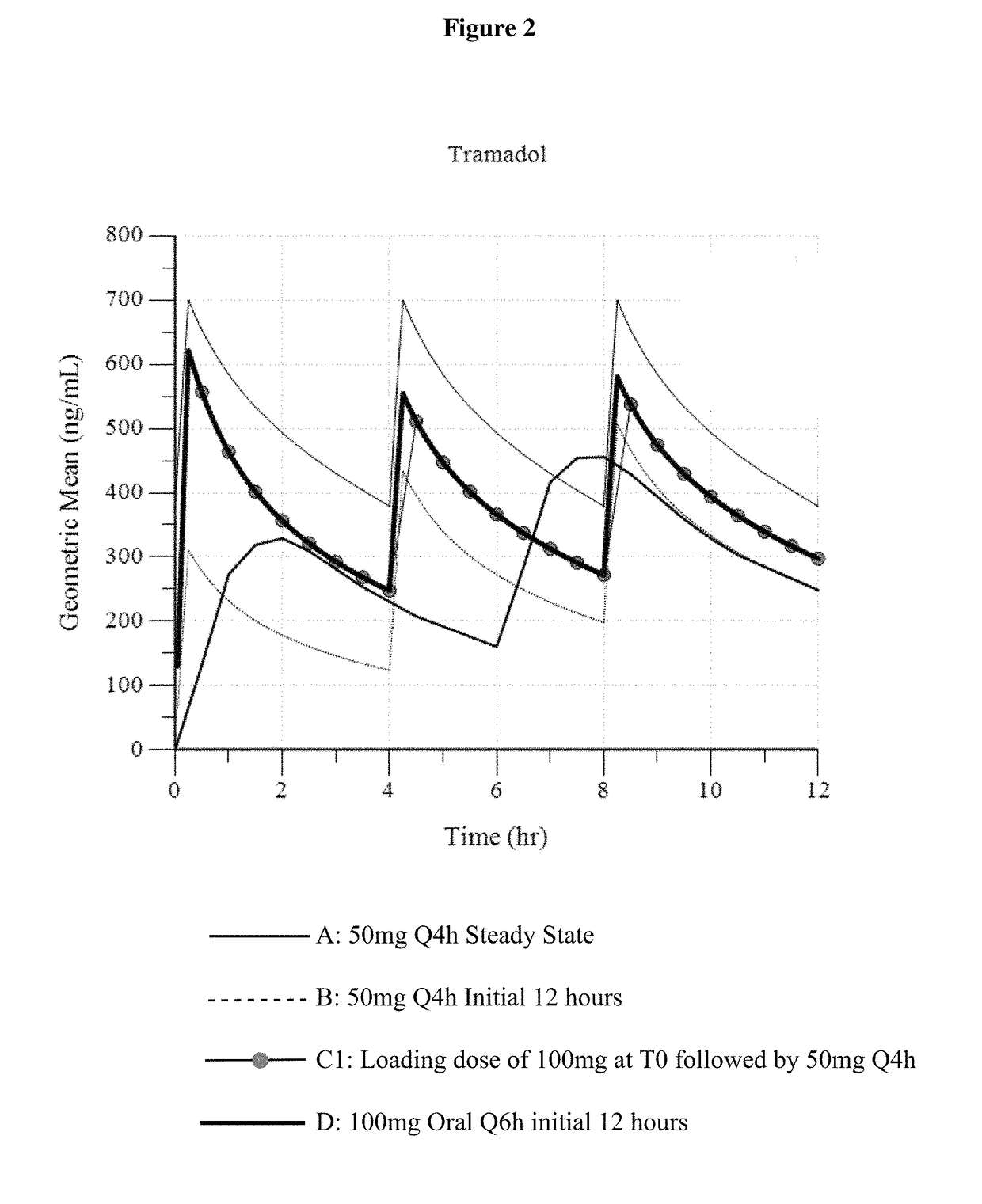
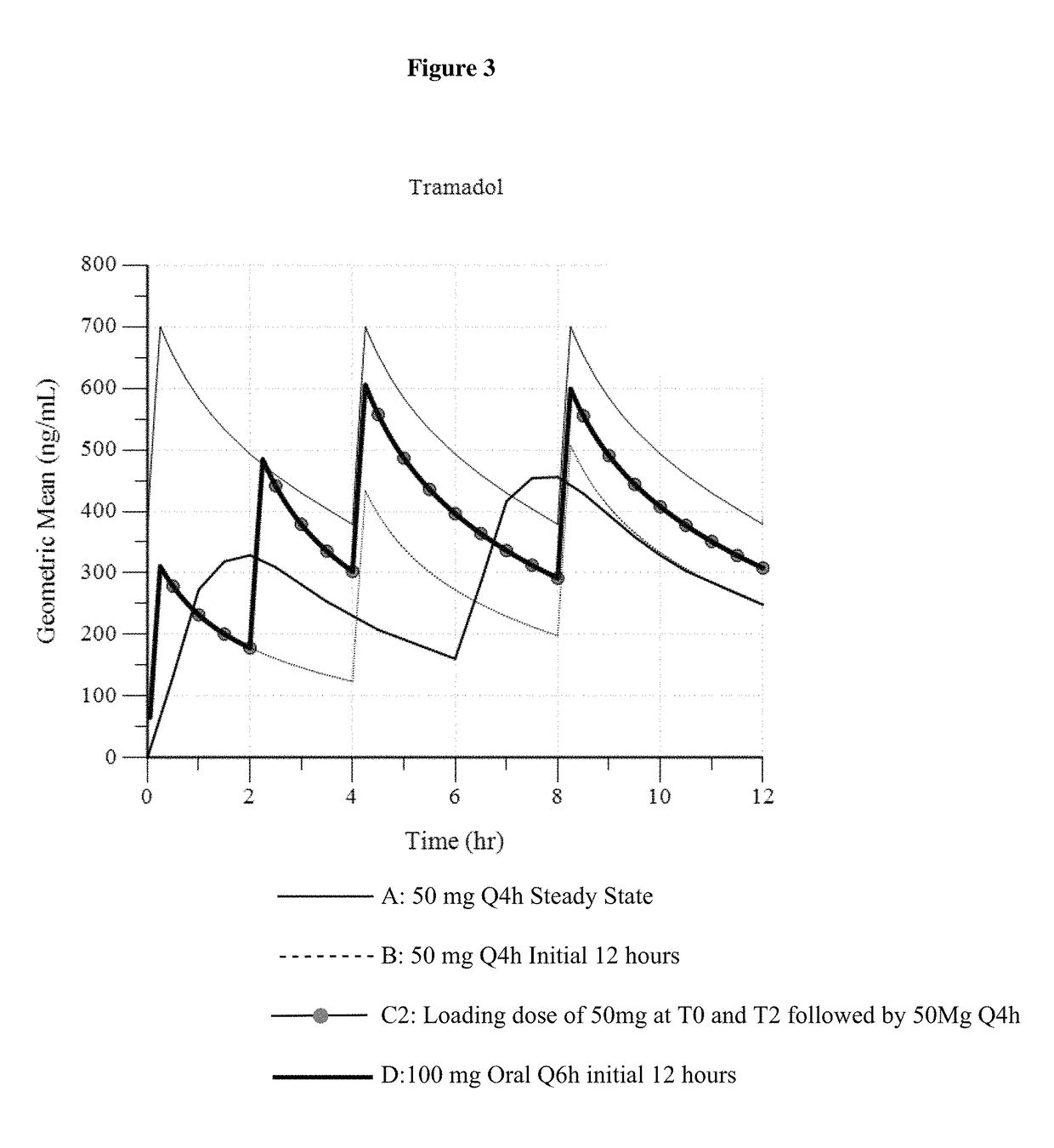
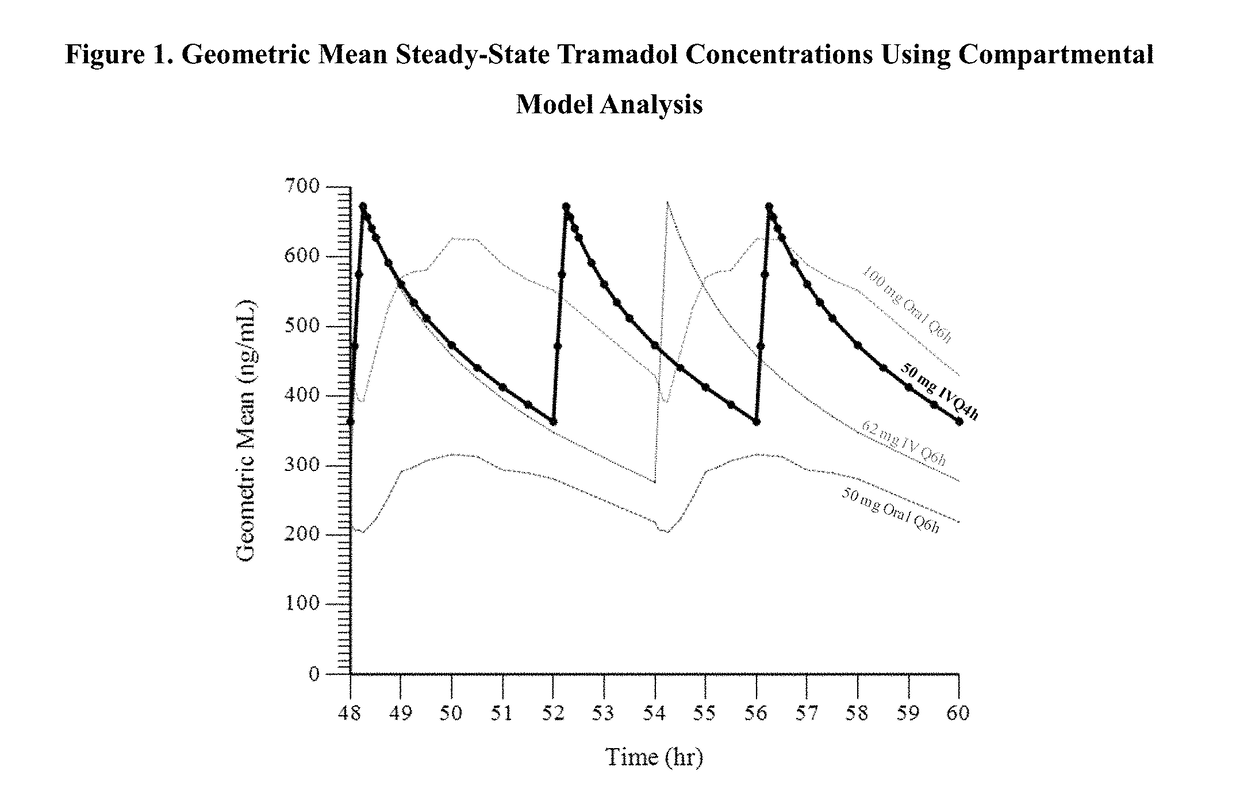
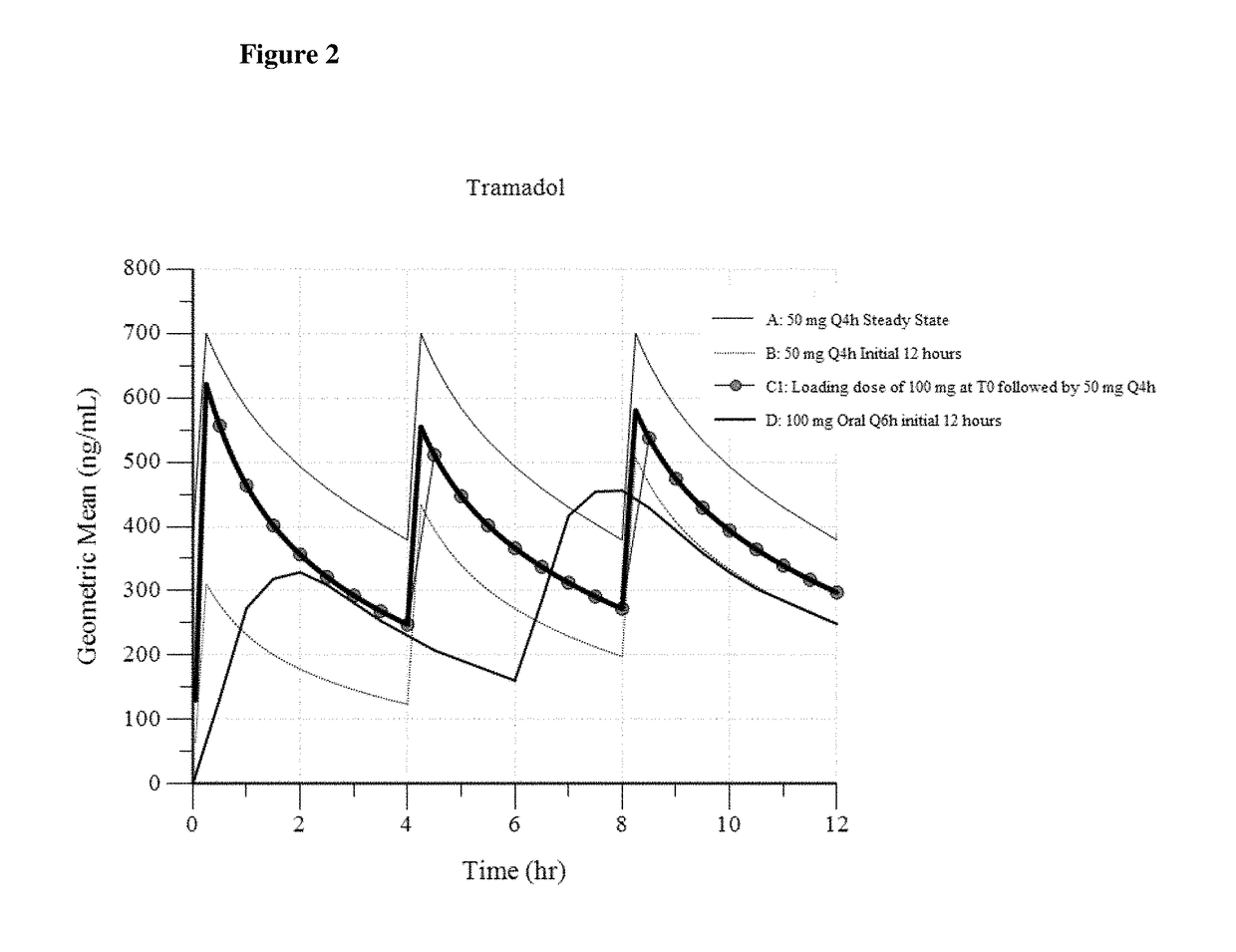
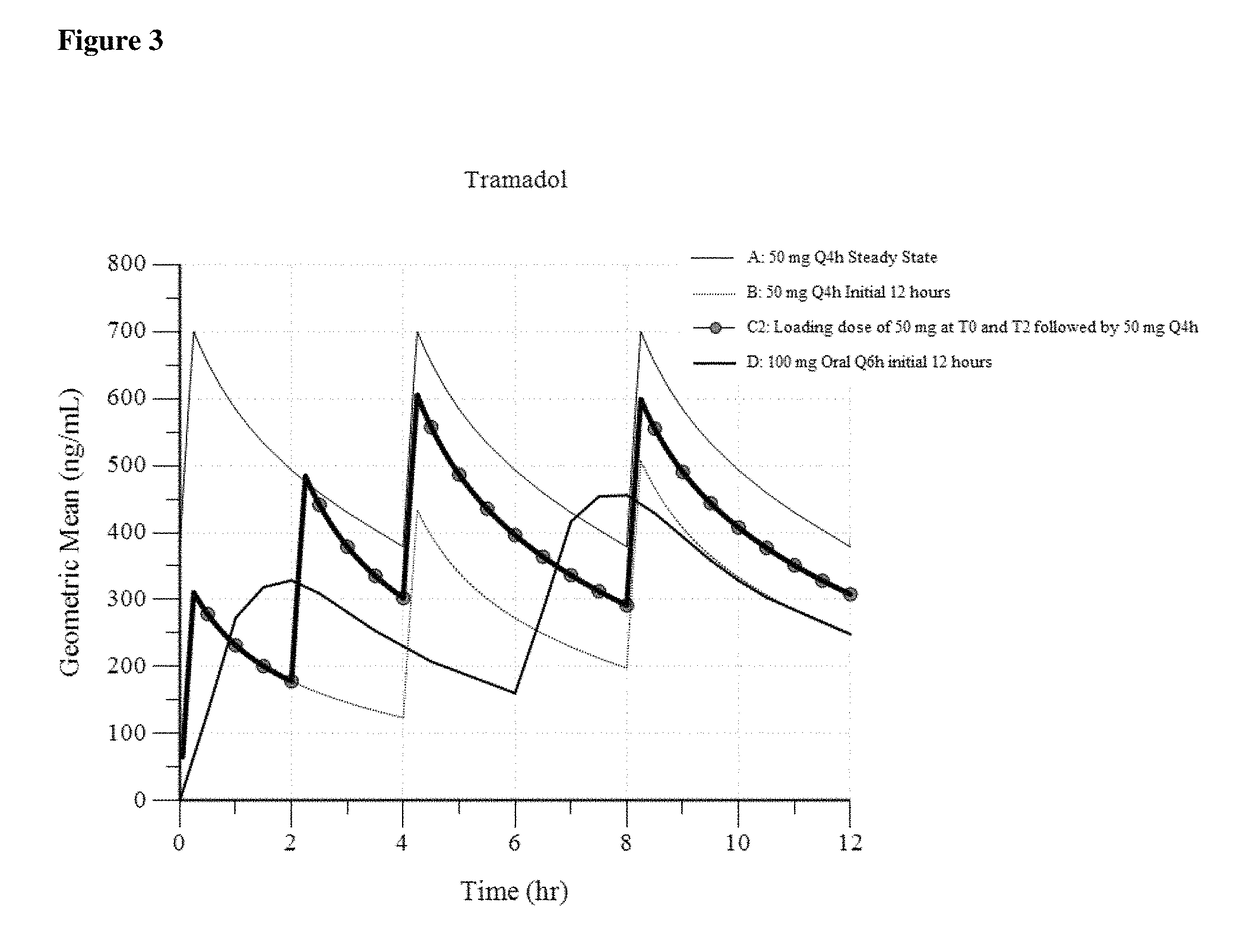
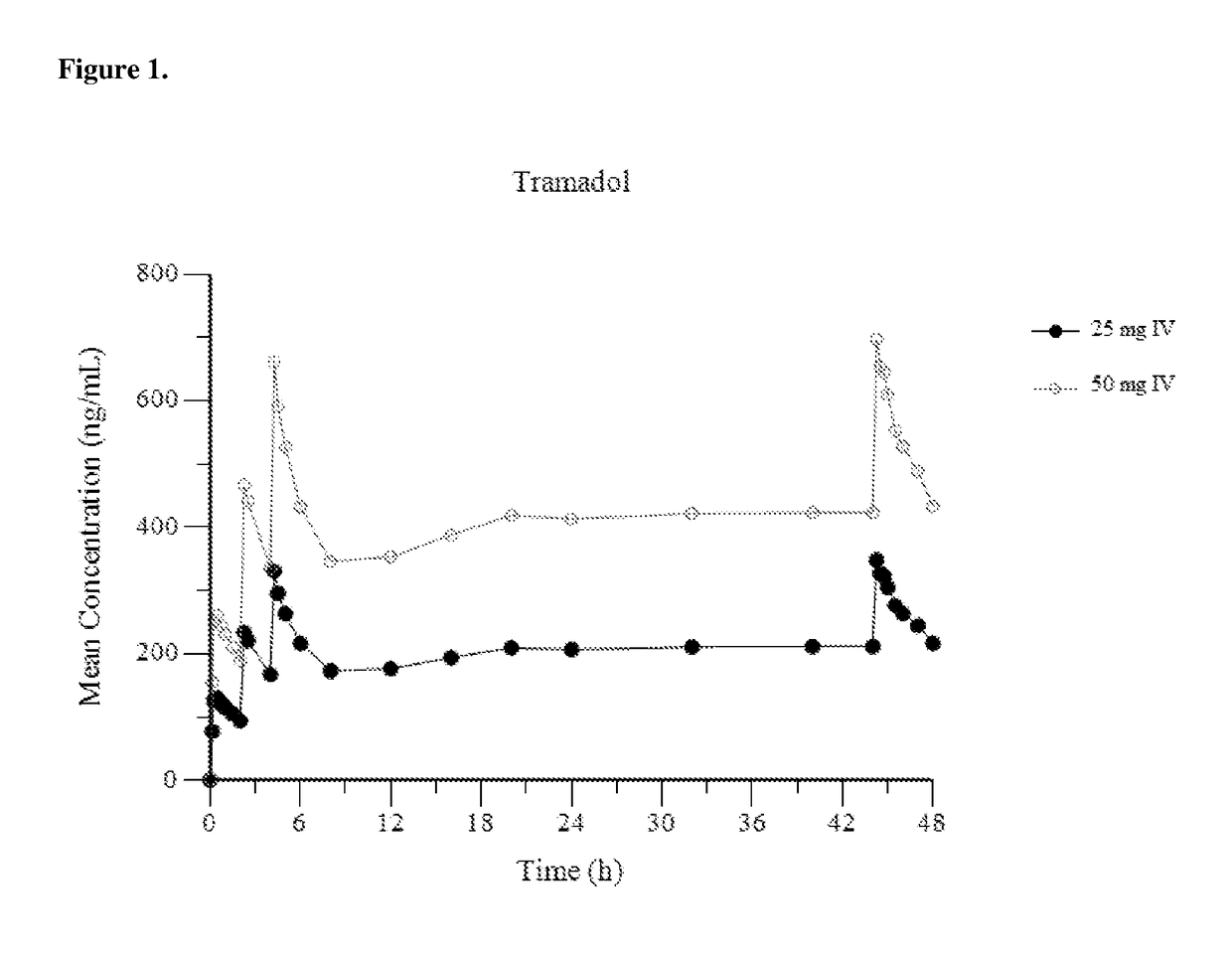
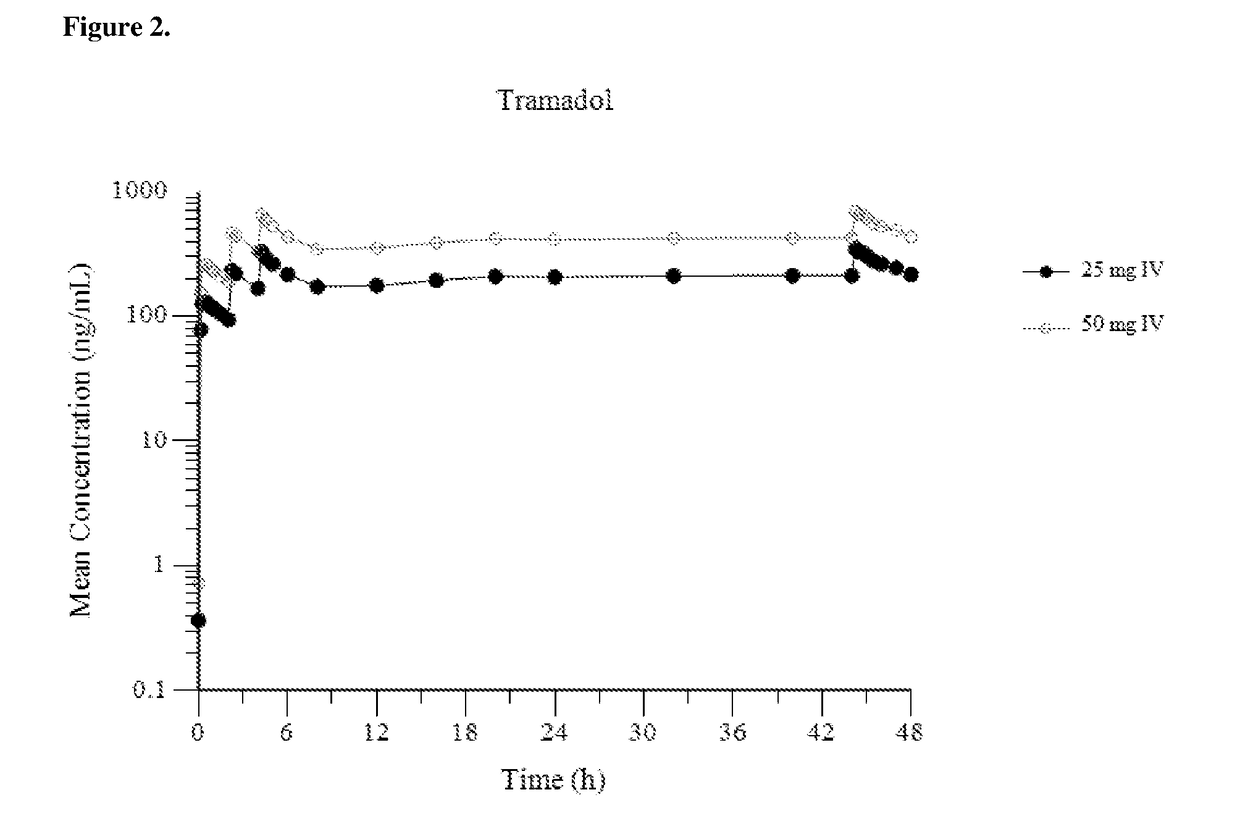
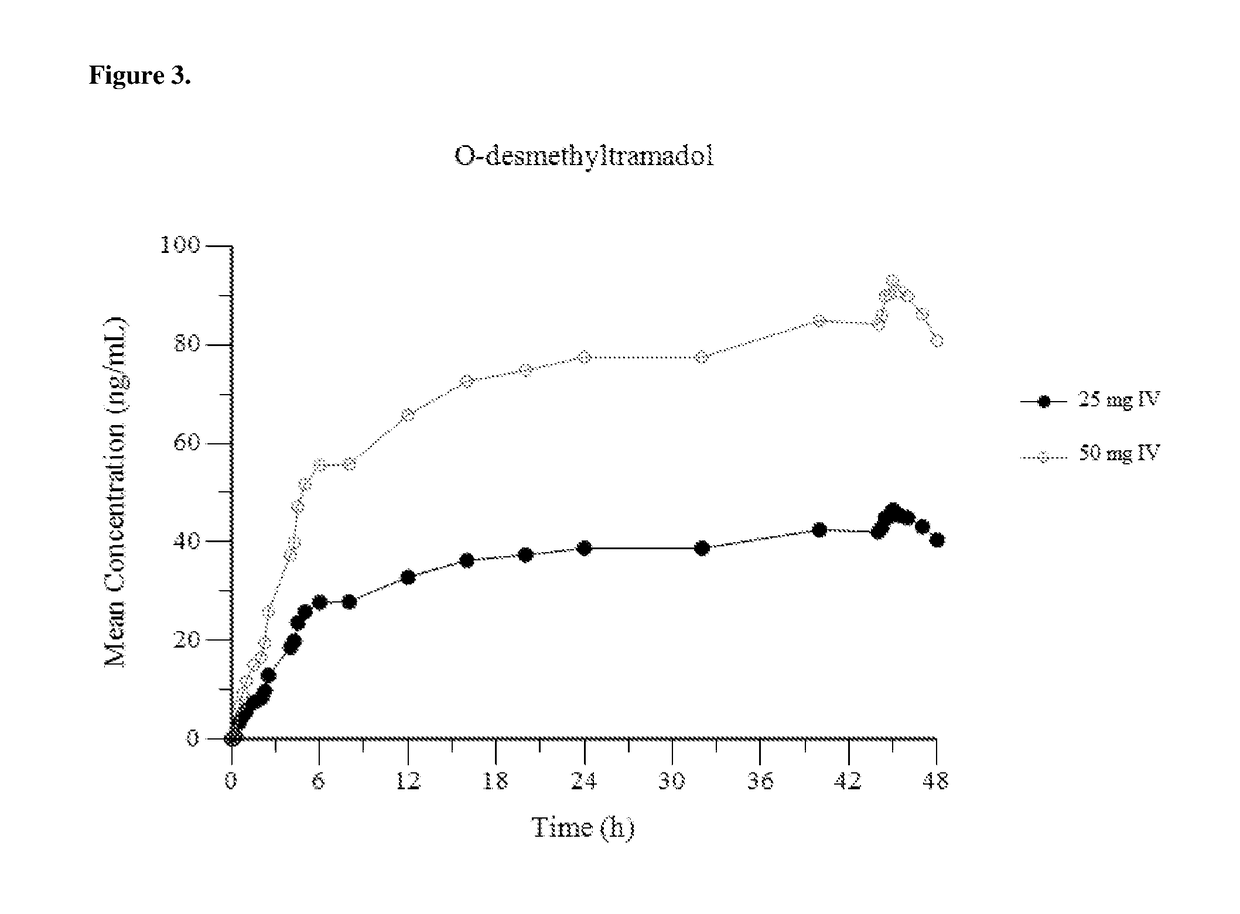
Intravenous administration of tramadol
ActiveUS9693949B1InhibitionRelieve painOrganic active ingredientsNervous disorderDosing regimenRegimen
A method of treating pain, e.g., acute post-operative pain, by administering to a human patient(s) a therapeutically effective dose of tramadol intravenously in a dosing regimen which includes one or more loading doses administered at shortened intervals as compared to dosing at steady-state is disclosed. In certain embodiments, the dose of tramadol is from about 45 mg to about 80 mg and the second (and optionally) third doses are intravenously administered at intervals of from about 2 to about 3 hours, and thereafter the tramadol is intravenously administered at a dosing interval of about 4 to about 6 hours, until the patient no longer requires treatment with tramadol. In preferred embodiments, the intravenous dosing regimen provides a Cmax and AUC of tramadol is similar to the Cmax and AUC of an oral dose of 100 mg tramadol HCl given every 6 hours. In certain preferred embodiments, the dosing regimen comprises 50 mg IV tramadol at Hour 0, followed by 50 mg at Hour 2, 50 mg at hour 4, and 50 mg every 4 hours thereafter (e.g., until the patient no longer requires treatment with tramadol).
Owner:REVOGENEX IRELAND
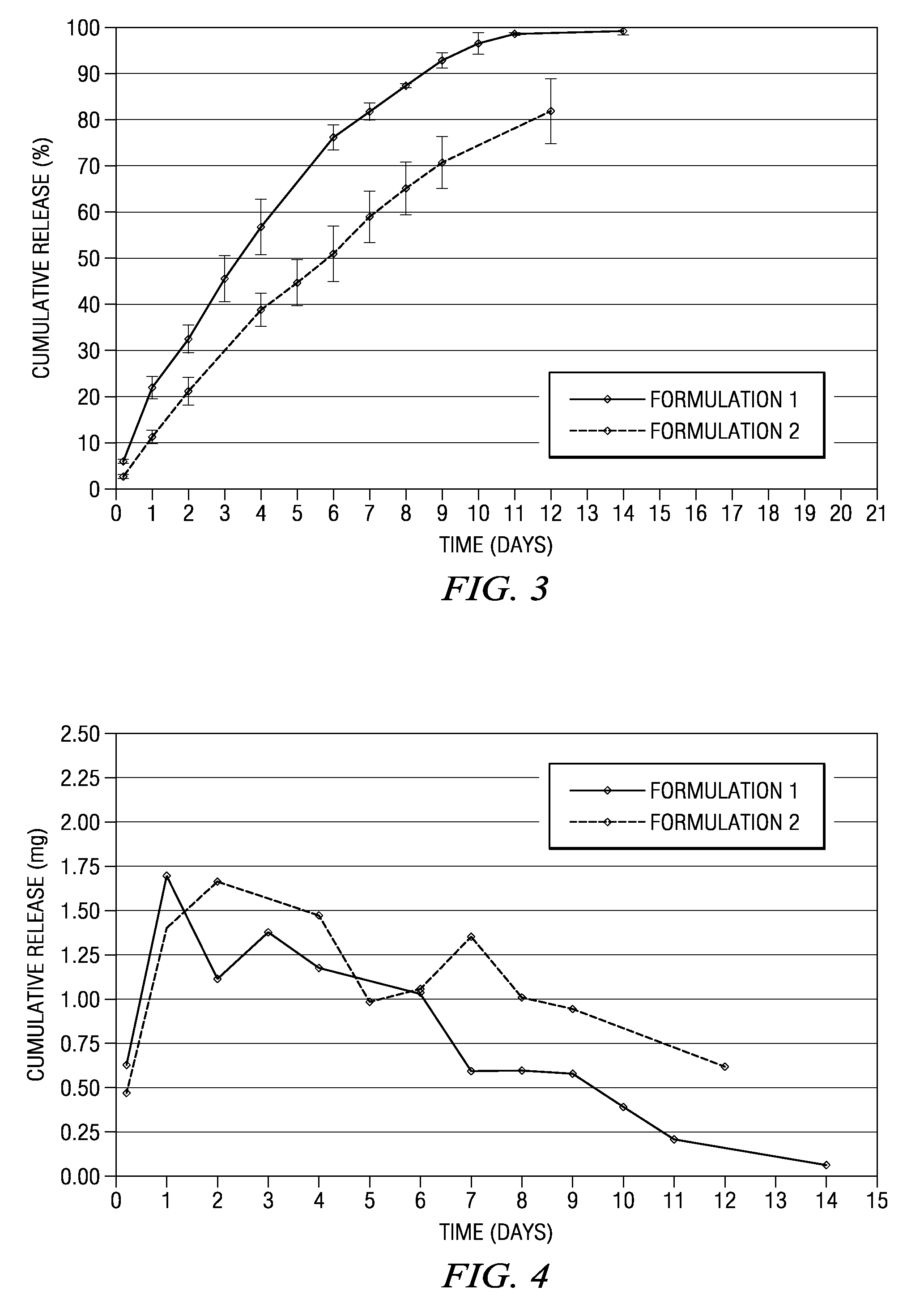
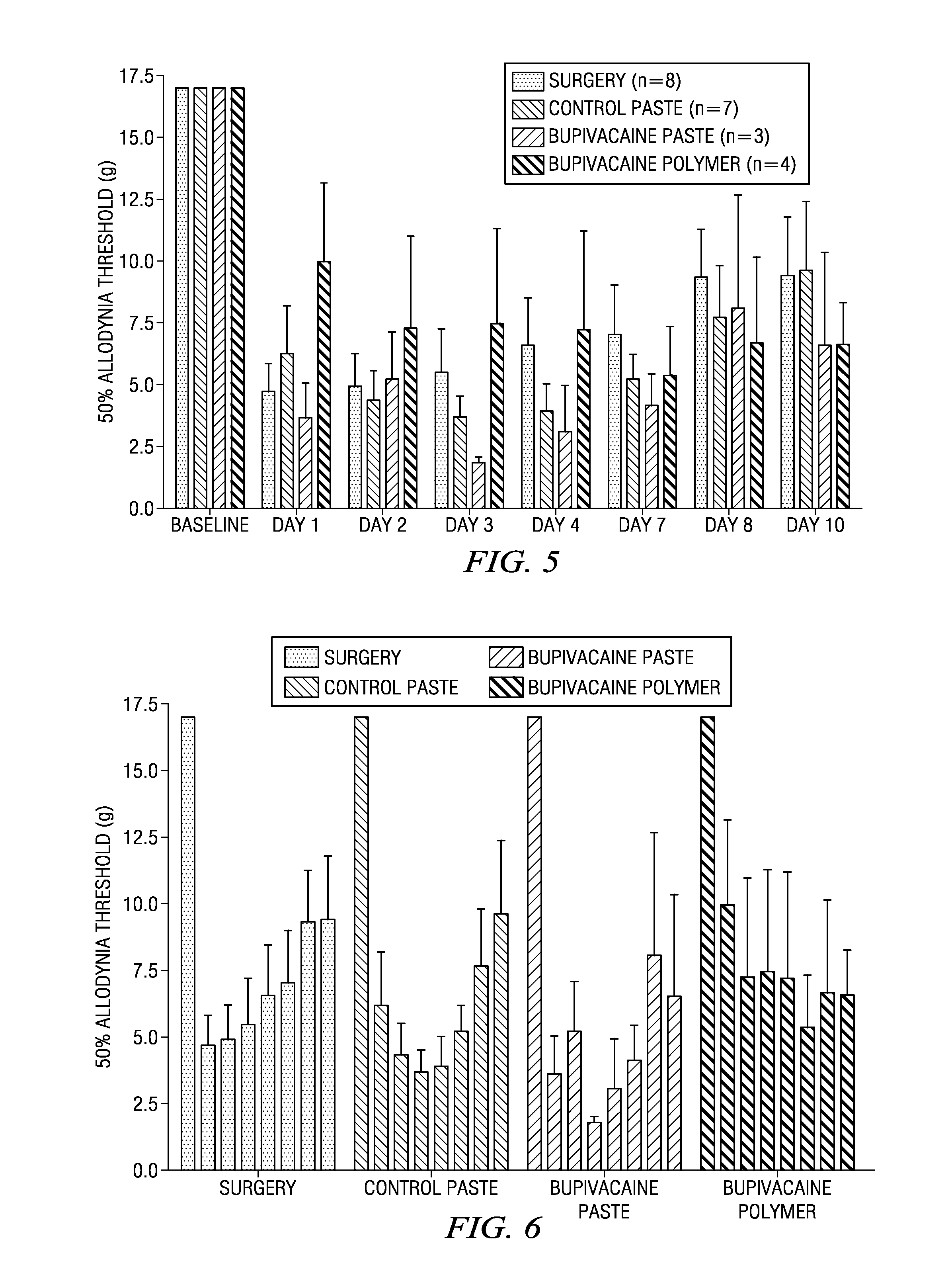
Methods and compositions for treating post-operative pain comprising a local anesthetic
ActiveUS20090264472A1Ease the pain of treatmentReduce deliveryPowder deliveryBiocidePharmacyPharmaceutical drug
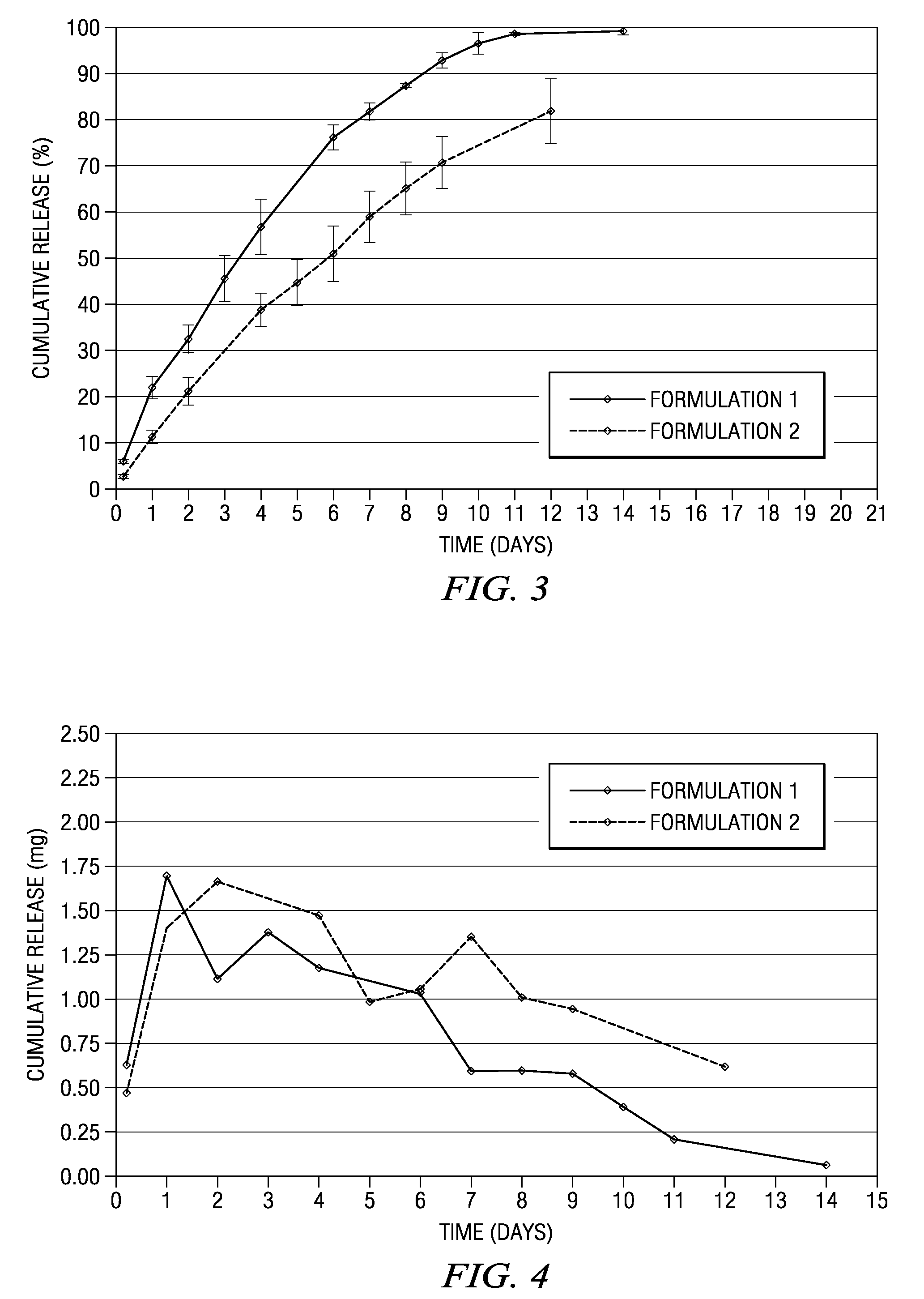
The present invention is directed to an implantable drug depot useful for reducing, preventing or treating post-operative pain in a patient in need of such treatment, the implantable drug depot comprising a polymer and a therapeutically effective amount of a local anesthetic or pharmaceutically acceptable salt thereof, wherein the drug depot is implantable at a site beneath the skin to reduce, prevent or treat post-operative pain, and the drug depot is capable of releasing (i) a bolus dose of the local anesthetic or pharmaceutically acceptable salt thereof at a site beneath the skin and (ii) a sustained release dose of an effective amount of the local anesthetic or pharmaceutically acceptable salt thereof over a period of at least 4 days.
Owner:COMPANION SPINE LLC +1
Intravenous administration of tramadol
ActiveUS9980900B2Rapid onsetRelieve painOrganic active ingredientsNervous disorderDosing regimenRegimen
Owner:REVOGENEX IRELAND
Long-acting polymeric delivery systems
ActiveUS20170035777A1Efficient releaseImprove the level ofAntipyreticAnalgesicsAnesthetic AgentDelivery vehicle
Compositions comprised of a delivery vehicle or delivery system and an active agent dispersed within the delivery vehicle or system, wherein the delivery vehicle or system contains a polyorthoester polymer and a polar aprotic solvent. Also disclosed are low viscosity delivery systems for administration of active agents. The low viscosity delivery systems have a polyorthoester polymer, a polar aprotic solvent and a solvent containing a triglyceride viscosity reducing agent. Compositions described include an amide- or anilide-type local anesthetic of the “caine” classification, and a non-steroidal anti-inflammatory drug (NSAID), along with related methods, e.g., for treatment of post-operative pain or for prophylactic treatment of pain. The compositions are suitable for delivery via, e.g., direct application and instillation, intradermal injection, subcutaneous injection, and nerve block (perineural).
Owner:HERON THERAPEUTICS
Long-acting polymeric delivery systems
ActiveUS20160375140A1Improved profileEffective pain reliefPowder deliveryNervous disorderAnesthetic AgentActive agent
Compositions comprised of a delivery vehicle or delivery system and an active agent dispersed within the delivery vehicle or system, wherein the delivery vehicle or system contains a polyorthoester polymer and a polar aprotic solvent. Also disclosed are low viscosity delivery systems for administration of active agents. The low viscosity delivery systems have a polyorthoester polymer, a polar aprotic solvent and a solvent containing a triglyceride viscosity reducing agent. Compositions described include an amide- or anilide-type local anesthetic of the “caine” classification, and a non-steroidal anti-inflammatory drug (NSAID), along with related methods, e.g., for treatment of post-operative pain or for prophylactic treatment of pain. The compositions are suitable for delivery via, e.g., direct application and instillation, intradermal injection, subcutaneous injection, and nerve block (perineural).
Owner:HERON THERAPEUTICS
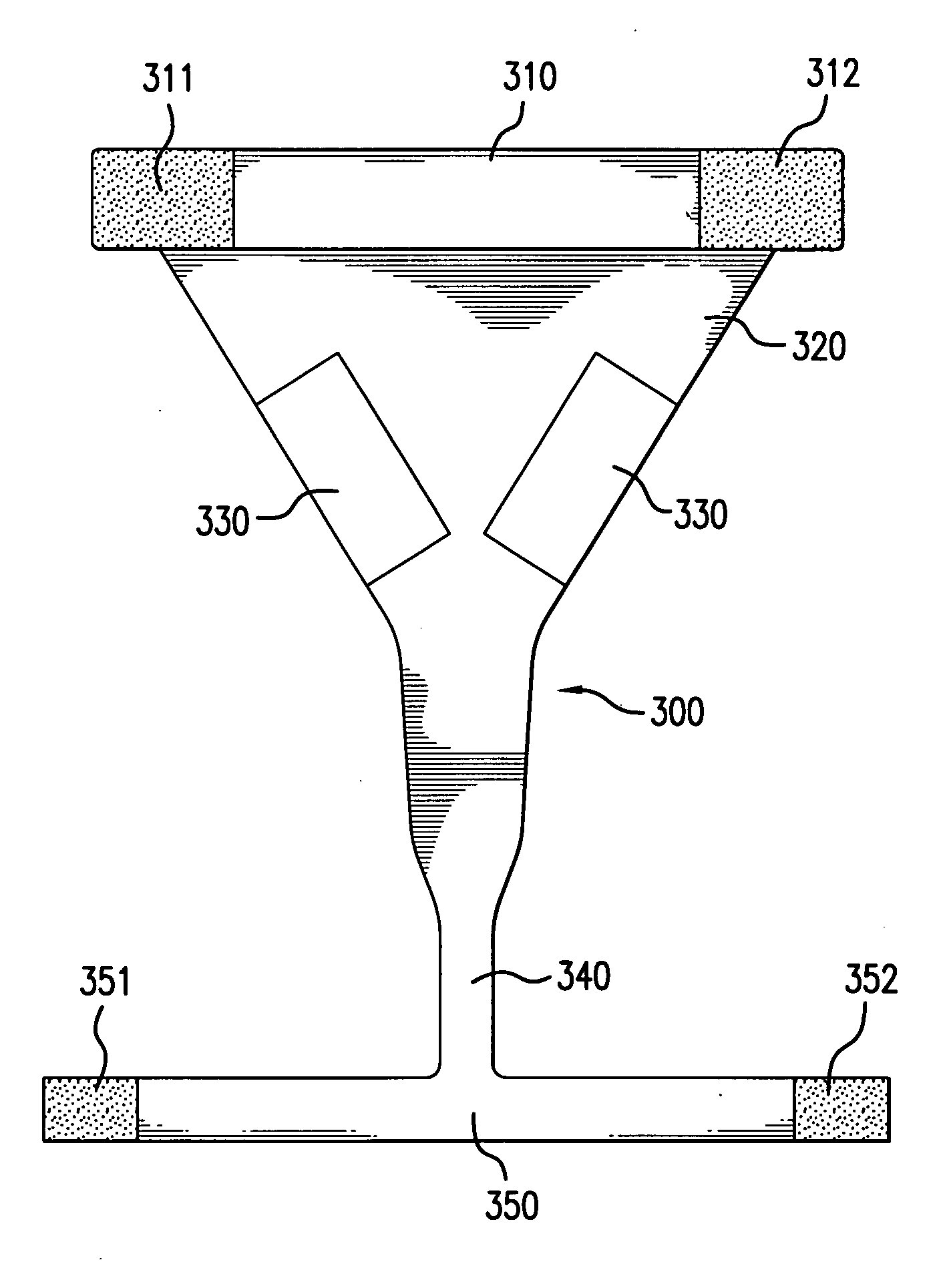
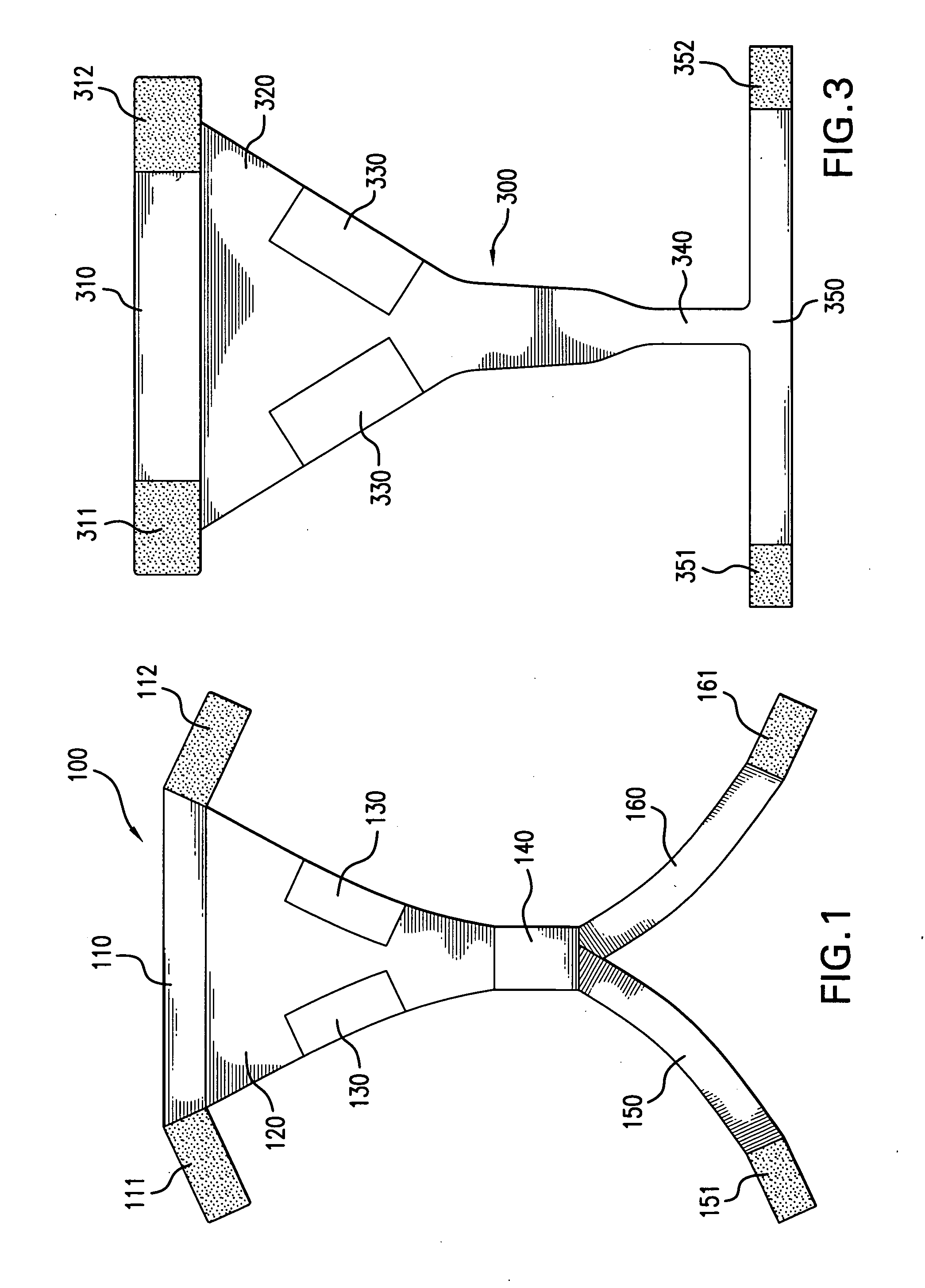
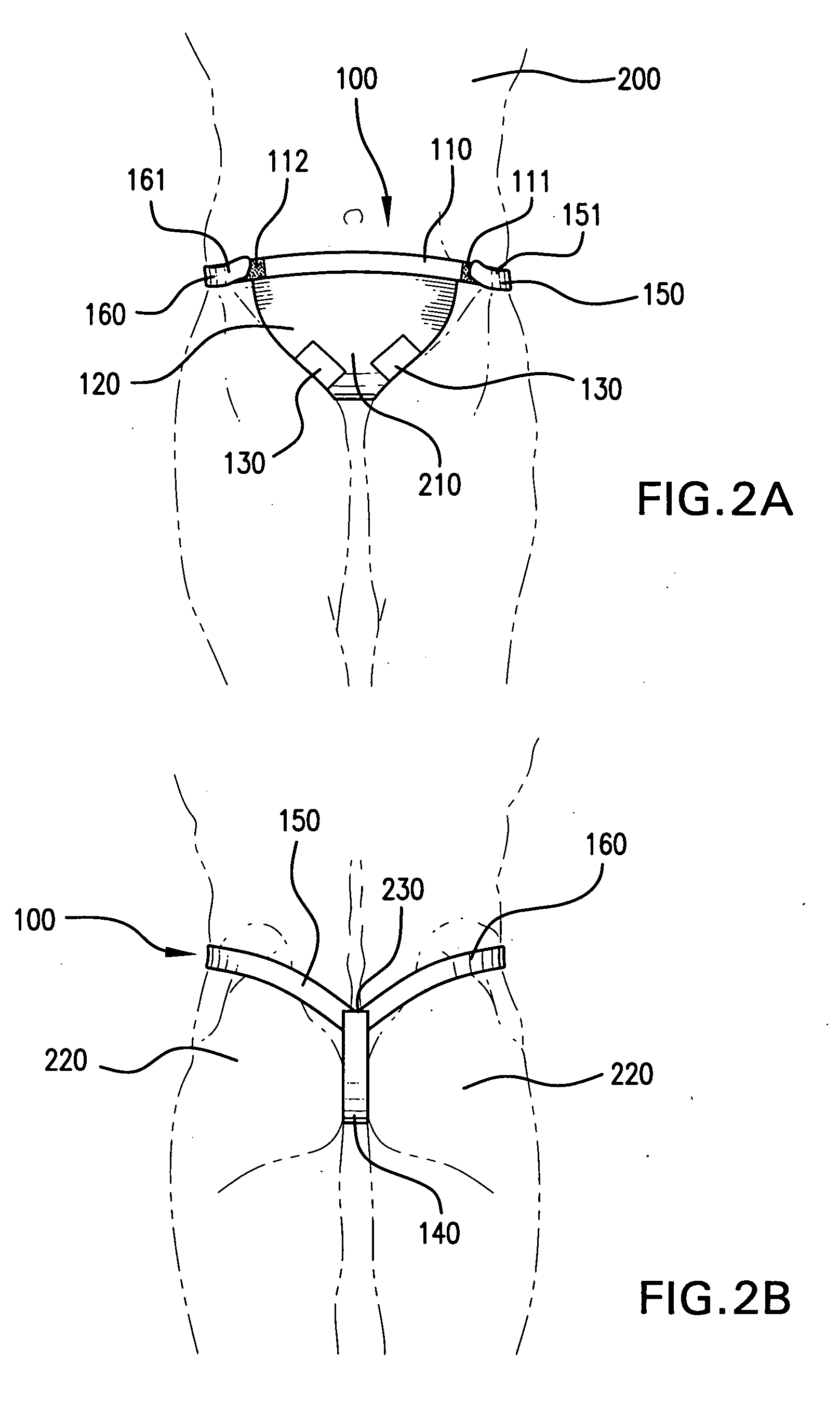
Methods & apparati for the close application of therapeutic & other devices to the pelvic area
ActiveUS20100094386A1Easy on/off characteristicEasy to placeTherapeutic coolingUndergarmentsPhysical therapyPostoperative pain
Owner:NFF INVENTIONS +1
Intravenous administration of tramadol
Owner:REVOGENEX IRELAND
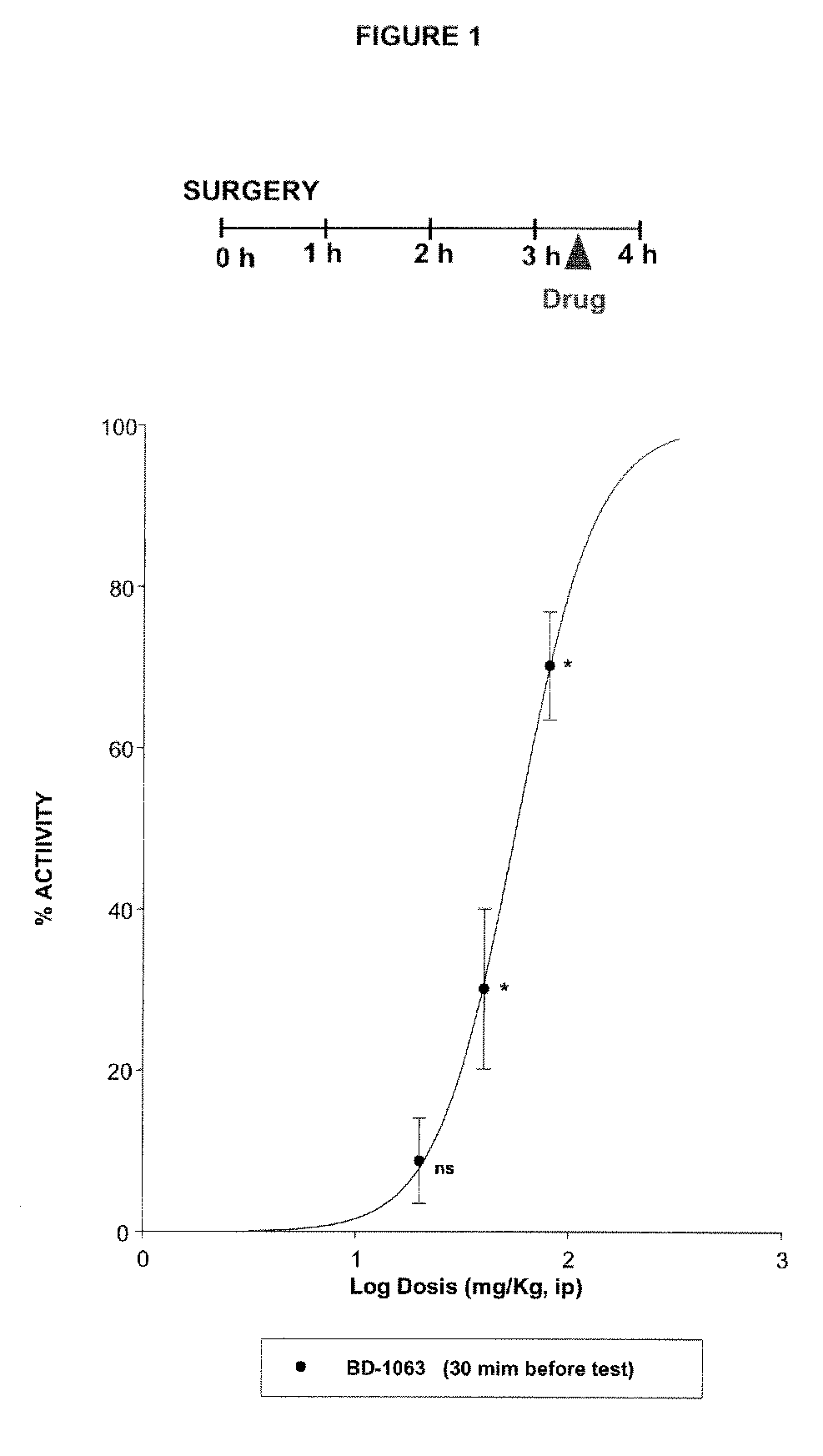
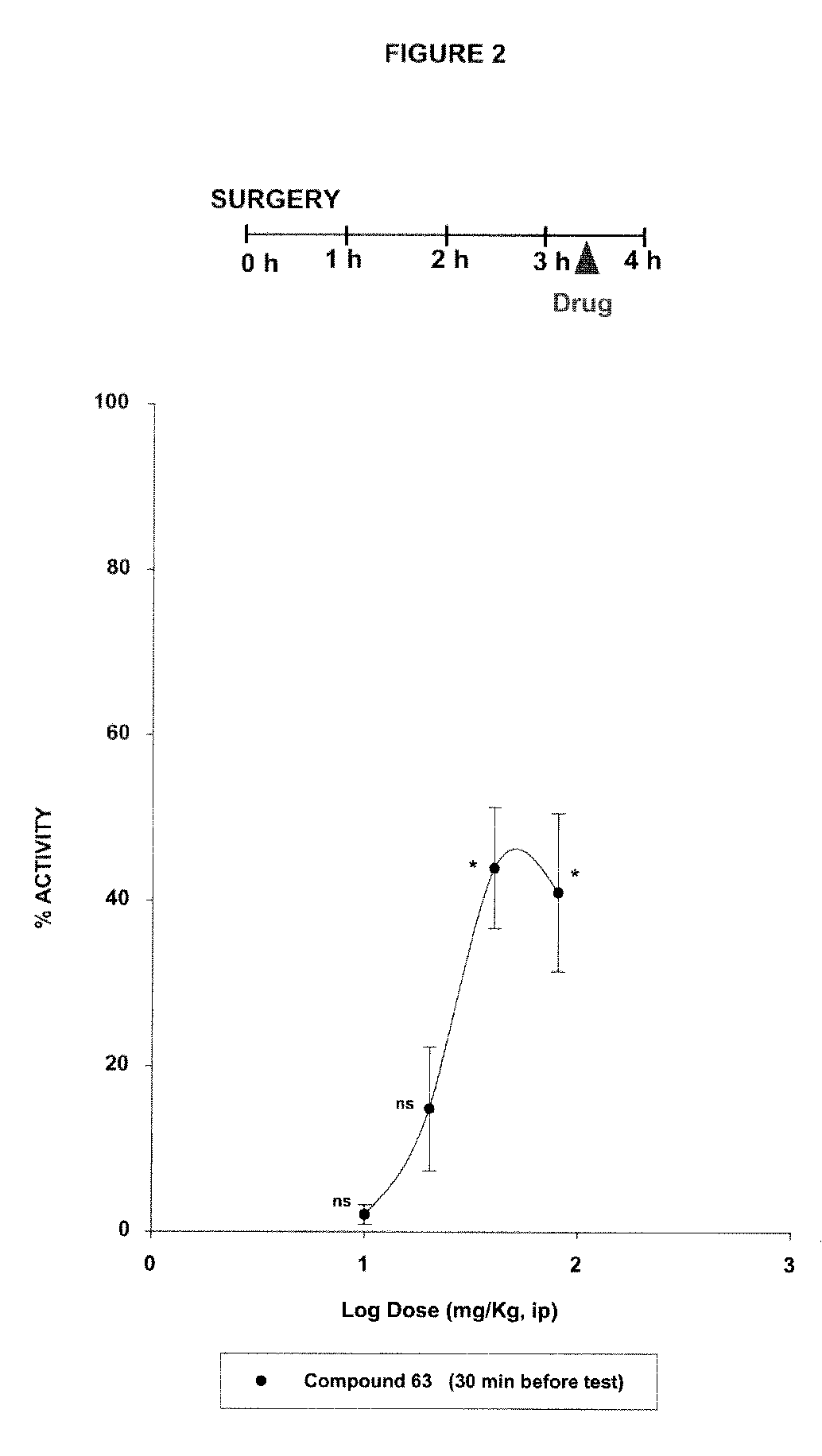
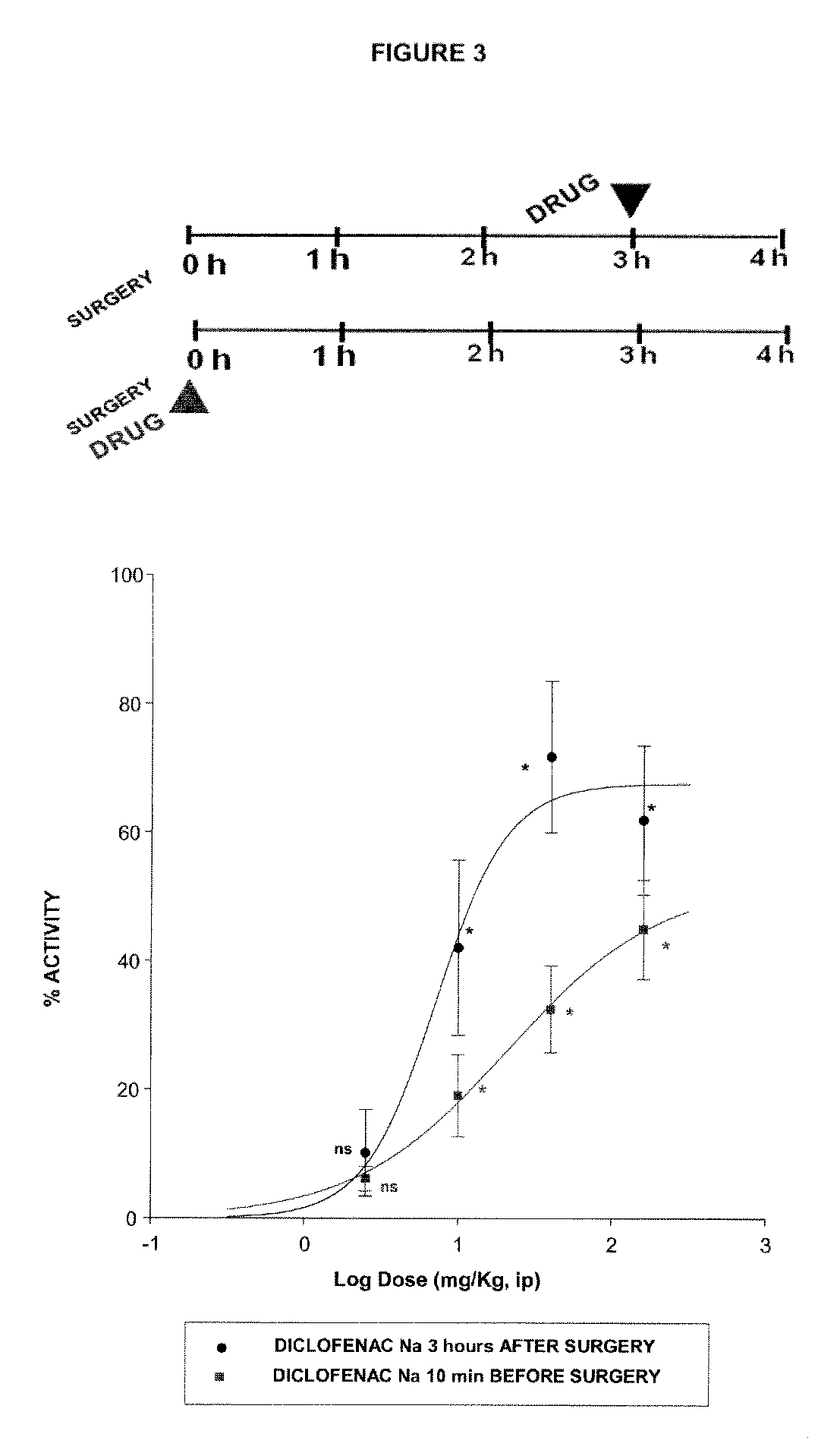
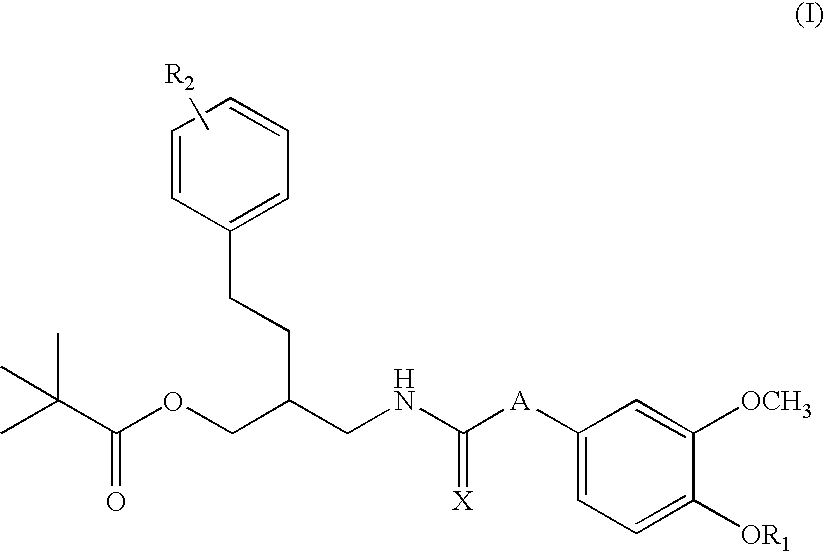
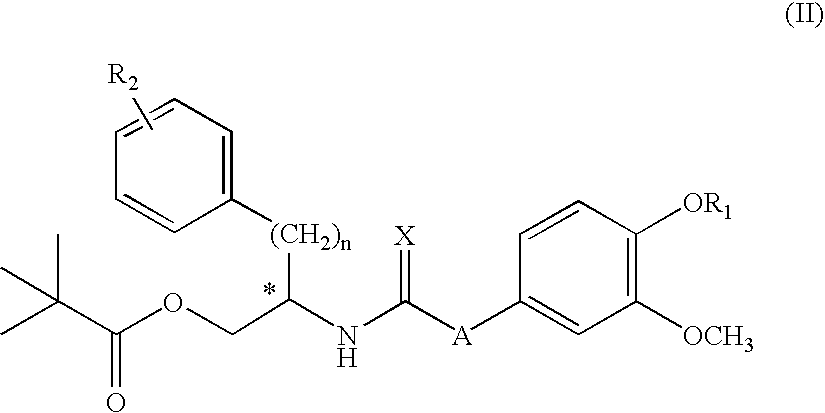
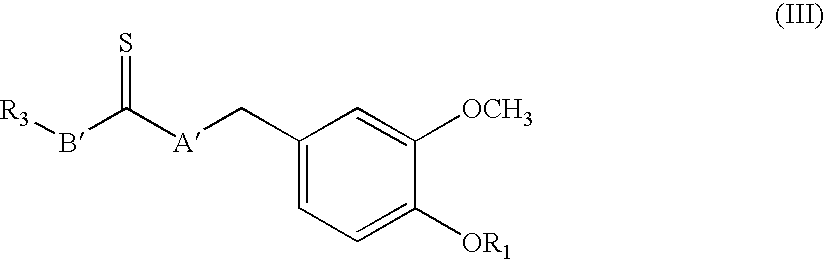
Sigma ligands for use in the prevention and/or treatment of post-operative pain
The invention refers to the use of a sigma ligand, particularly a sigma ligand of formulae (I), (II) or (III) to prevent and / or treat acute and chronic pain developed as a consequence of surgery, especially superficial and / or deep pain secondary to surgical tissue injury, and peripheral neuropathic pain, neuralgia, allodynia, causalgia, hyperalgesia, hyperesthesia, hyperpathia, neuritis or neuropathy secondary to surgical procedure.
Owner:LAB DEL DR ESTEVE SA
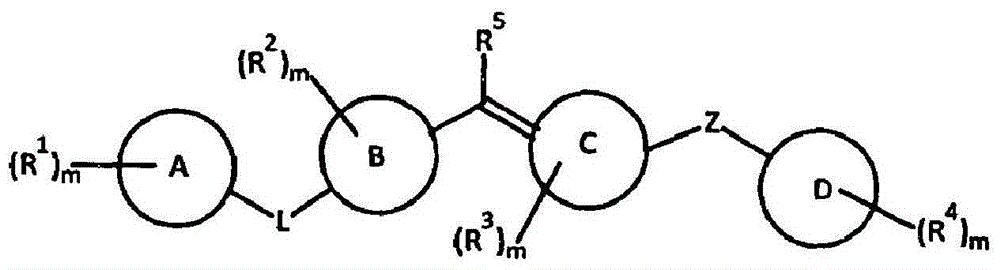
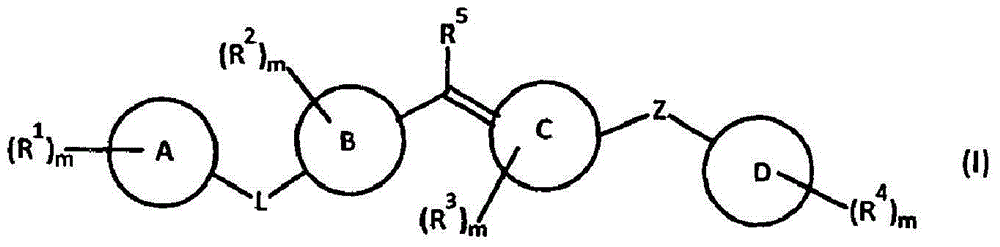
Amide compounds, compositions and applications thereof
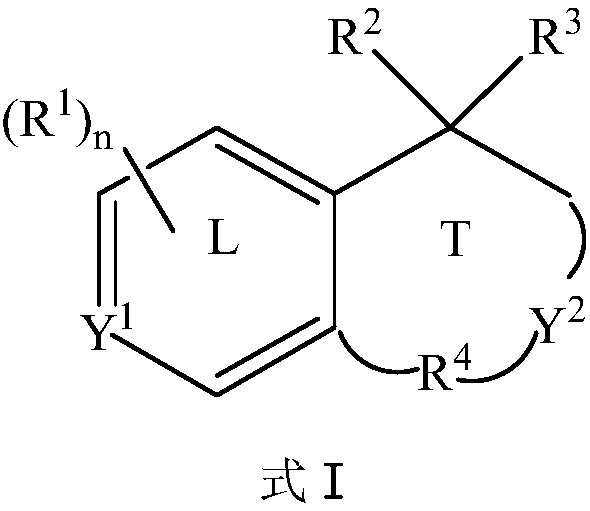
The present disclosure relates to substituted amide compounds that are inhibitors of Fatty Acid Amide Hydrolase (FAAH), their stereoisomers, tautomers, prodrugs, polymorphs, solvates, pharmaceutically acceptable salts, and pharmaceutical compositions containing them. These compounds are useful in the treatment, prevention, prophylaxis, management, or adjunct treatment of all medical conditions related to inhibition of Fatty Acid Amide Hydrolase (FAAH), such as pain including acute and post operative pain, chronic pain, cancer pain, cancer chemotherapy induced pain, neuropathic pain, nociceptive pain, inflammatory pain, back pain, pain due to disease of various origin such as: diabetic neuropathy, neurotropic viral disease including human immunodeficient virus (HIV), herpes zoster such as post herpetic neuralgia; polyneuropathy, neurotoxicity, mechanical nerve injury, carpal tunnel syndrome, immunologic mechanisms like multiple sclerosis; sleep disorders, anxiety and depression disorders, inflammatory disorders, weight and eating disorders, Parkinson's disease, addiction, spasticity, hypertension or other disorders. The disclosure also relates to the process of preparation of the amide compounds. Formula (1). The present disclosure also relates to methods for the preparation of such compounds, and to pharmaceutical compositions containing them.
Owner:ADVINUS THERAPEUTICS PVT LTD
Novel thiourea derivatives and the pharmaceutical compositions containing the same
ActiveUS20080064687A1Reduce usageUseful in treatmentUrea derivatives preparationBiocideIrritationJoint arthralgia
The present invention relates to novel thiourea derivatives as a modulator for vanilloid receptor (VR) and the pharmaceutical compositions containing the same. As diseases associated with the activity of vanilloid receptor, pain acute pain, chronic pain, neuropathic pain, post-operative pain, migraine, arthralgia, neuropathies, nerve injury, diabetic neuropathy, neurodegeneration, neurotic skin disorder, stroke, urinary bladder hypersensitiveness, irritable bowel syndrome, a respiratory disorder such as asthma or chronic obstructive pulmonary disease, irritation of skin, eye or mucous membrane, fervescence, stomach-duodenal ulcer, inflammatory bowel disease and inflammatory diseases can be enumerated. The present invention provides a pharmaceutical composition for prevention or treatment of these diseases.
Owner:PACIFIC
Methods of treating pain
InactiveUS20140221490A1Reduce pain intensityReduce the amount requiredOrganic active ingredientsBiocideSubject matterTreatment pain
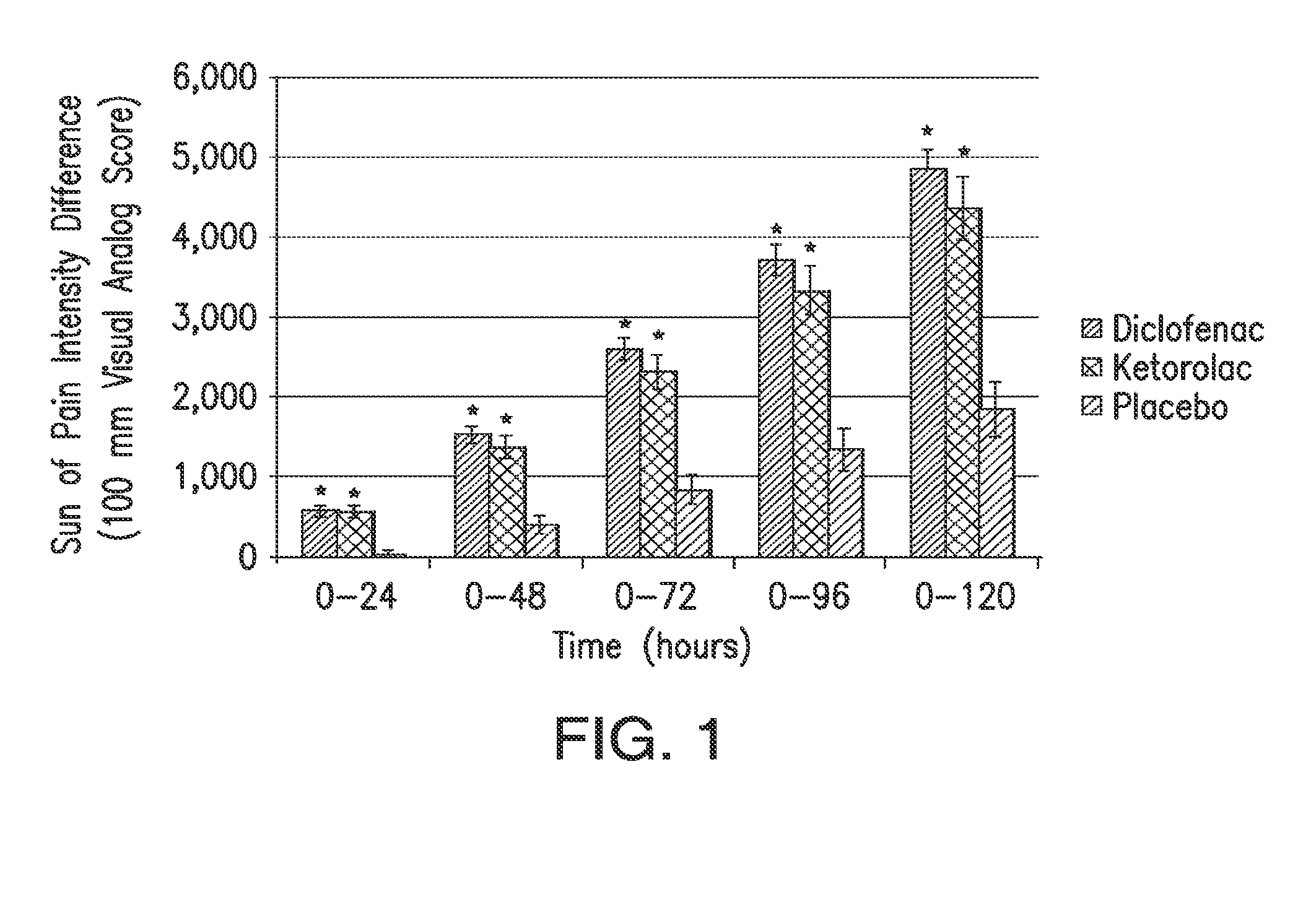
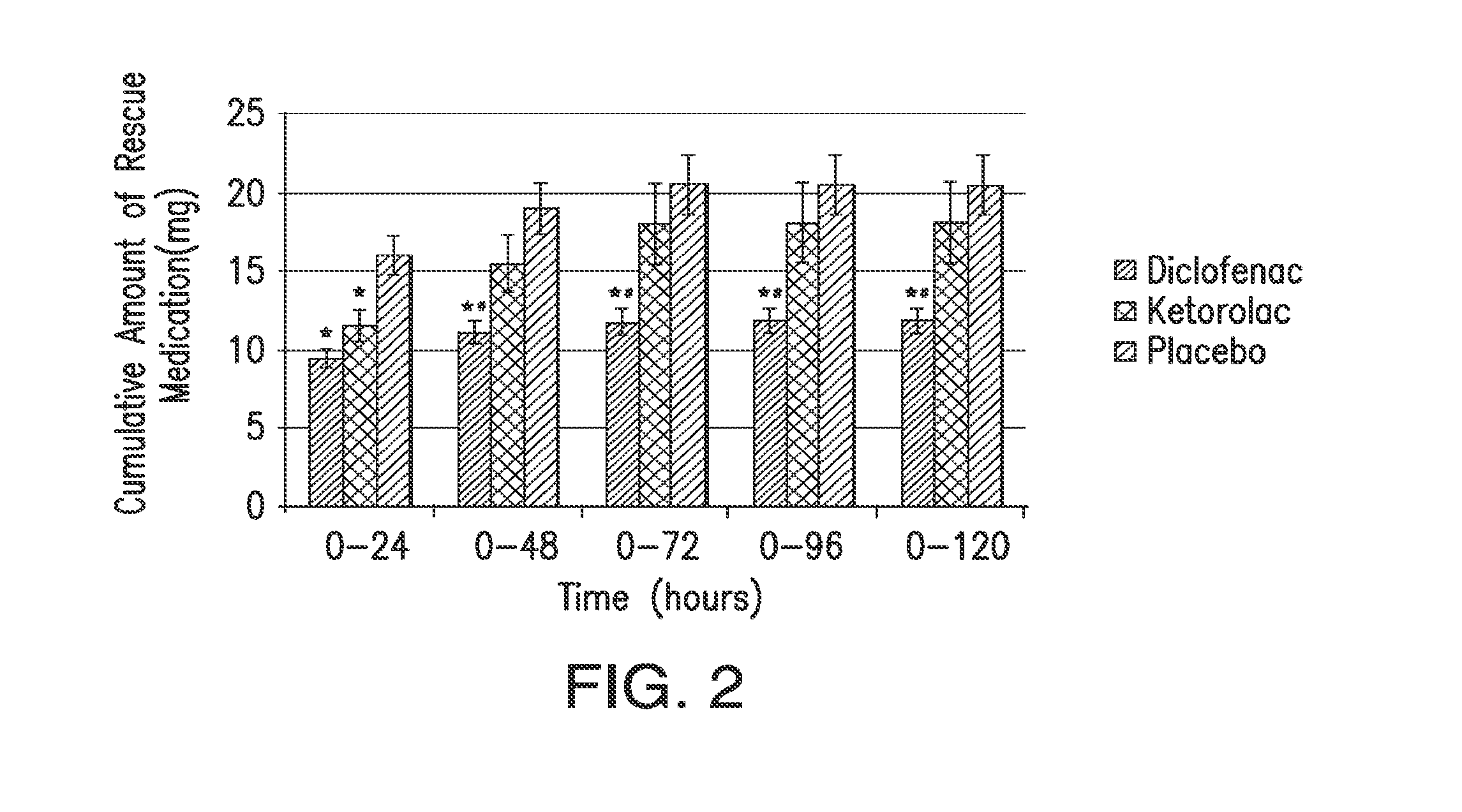
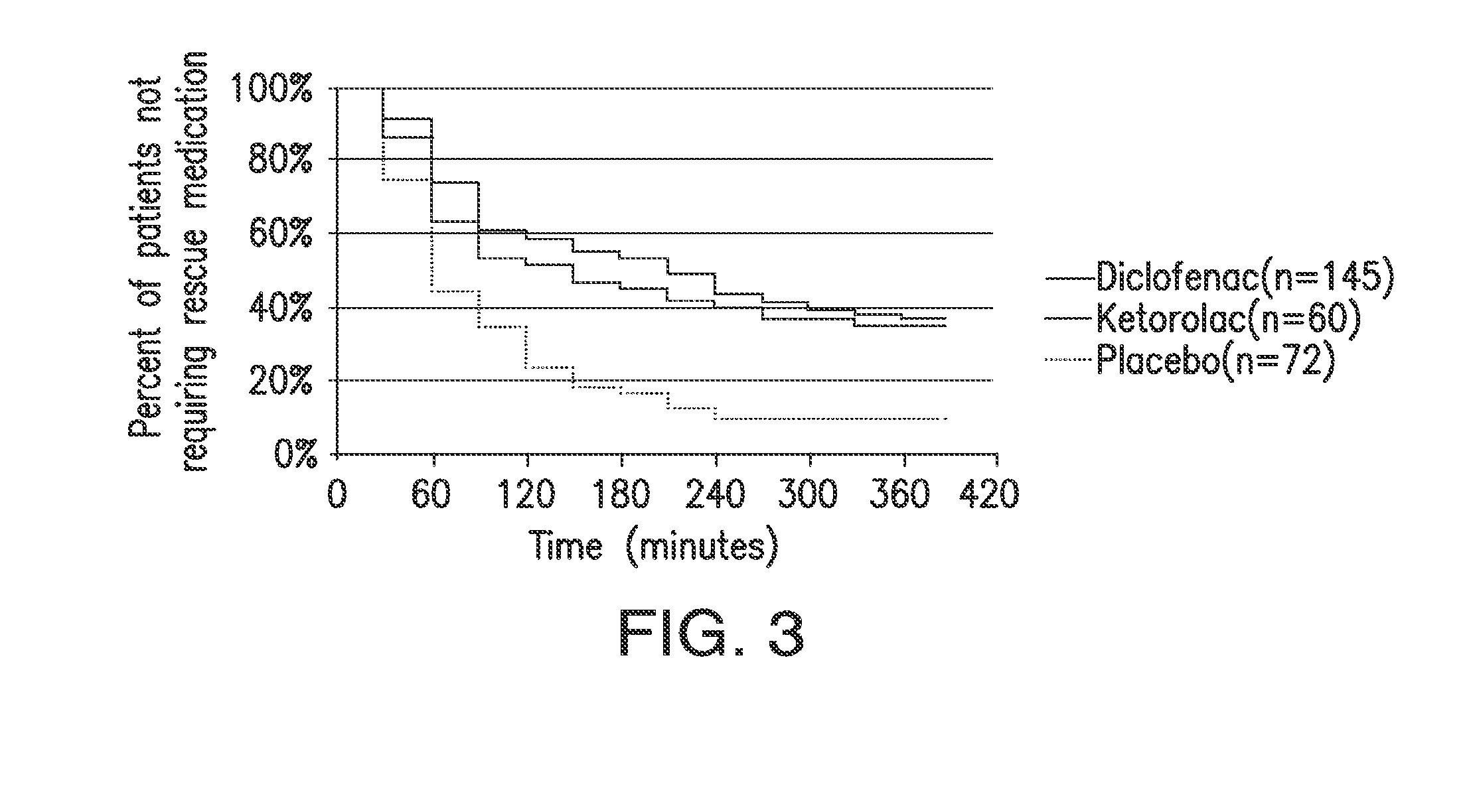
The presently disclosed subject matter is directed to methods of treating post-operative pain by administering a combination of a lower than minimum approved dose of diclofenac and beta-cyclodextrin. The presently disclosed subject matter is also directed to methods of treating pain in high risk or obese mammals in need of analgesia.
Owner:HOSPIRA
Methods and compositions for treating post-operative pain comprising a local anesthetic
ActiveUS8846068B2Easily allow accurate and precise implantation of a drug depotMinimal physical and psychological trauma to a patientPowder deliveryBiocideAnesthetic AgentPolymer
The present invention is directed to an implantable drug depot useful for reducing, preventing or treating post-operative pain in a patient in need of such treatment, the implantable drug depot comprising a polymer and a therapeutically effective amount of a local anesthetic or pharmaceutically acceptable salt thereof, wherein the drug depot is implantable at a site beneath the skin to reduce, prevent or treat post-operative pain, and the drug depot is capable of releasing (i) a bolus dose of the local anesthetic or pharmaceutically acceptable salt thereof at a site beneath the skin and (ii) a sustained release dose of an effective amount of the local anesthetic or pharmaceutically acceptable salt thereof over a period of at least 4 days.
Owner:COMPANION SPINE LLC +1
Methods and compositions for treating post-operative pain comprising clonidine
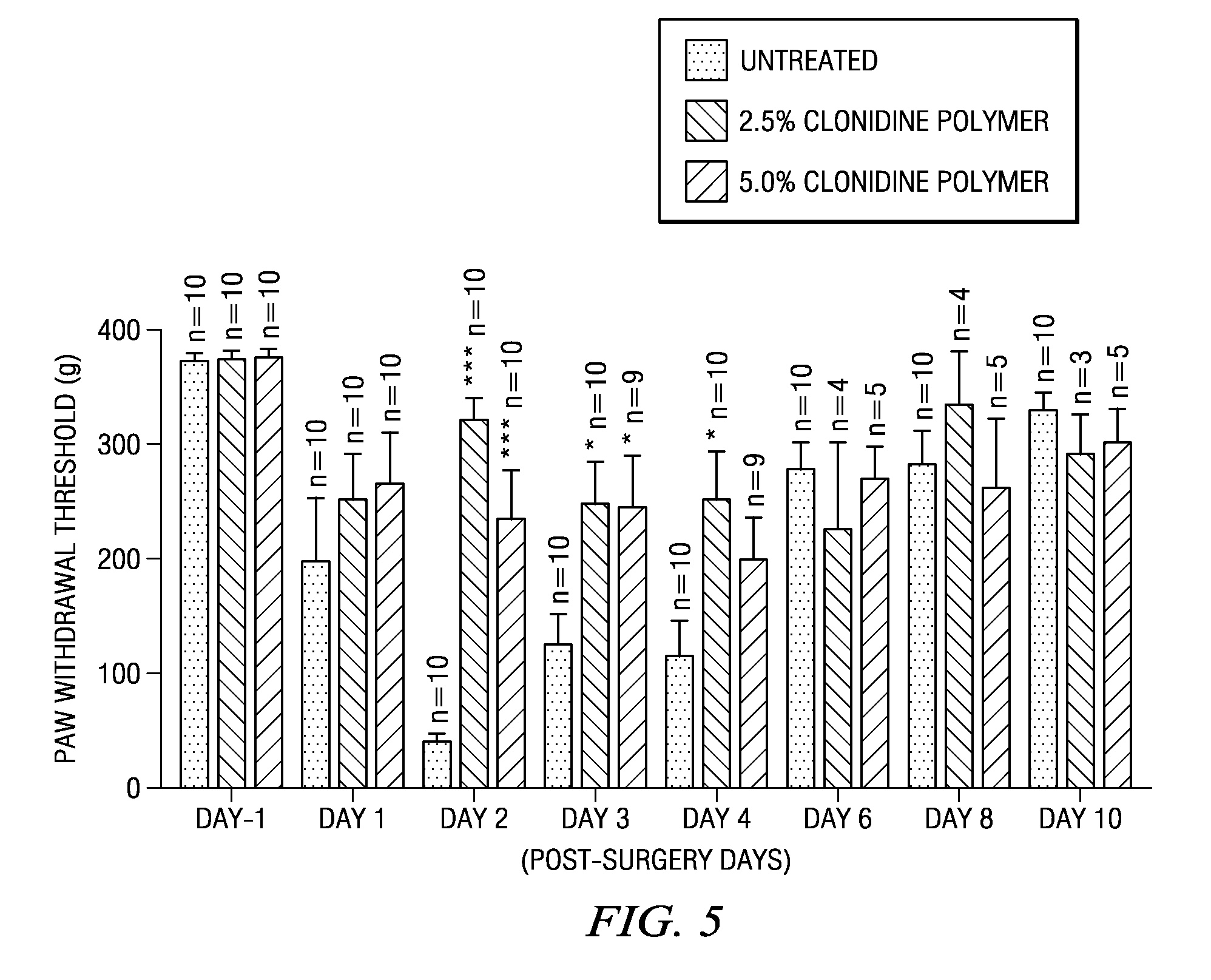
ActiveUS8629172B2Easily allow accurate and precise implantation of a drug depotMinimal physical and psychological trauma to a patientOrganic active ingredientsBiocidePharmaceutical drugEngineering
Owner:COMPANION SPINE LLC +1
Treatment of pain with gap junction modulation compounds
InactiveUS20110223204A1Easy to manageImprove solubilityOrganic active ingredientsNervous disorderDiseaseJoint pain
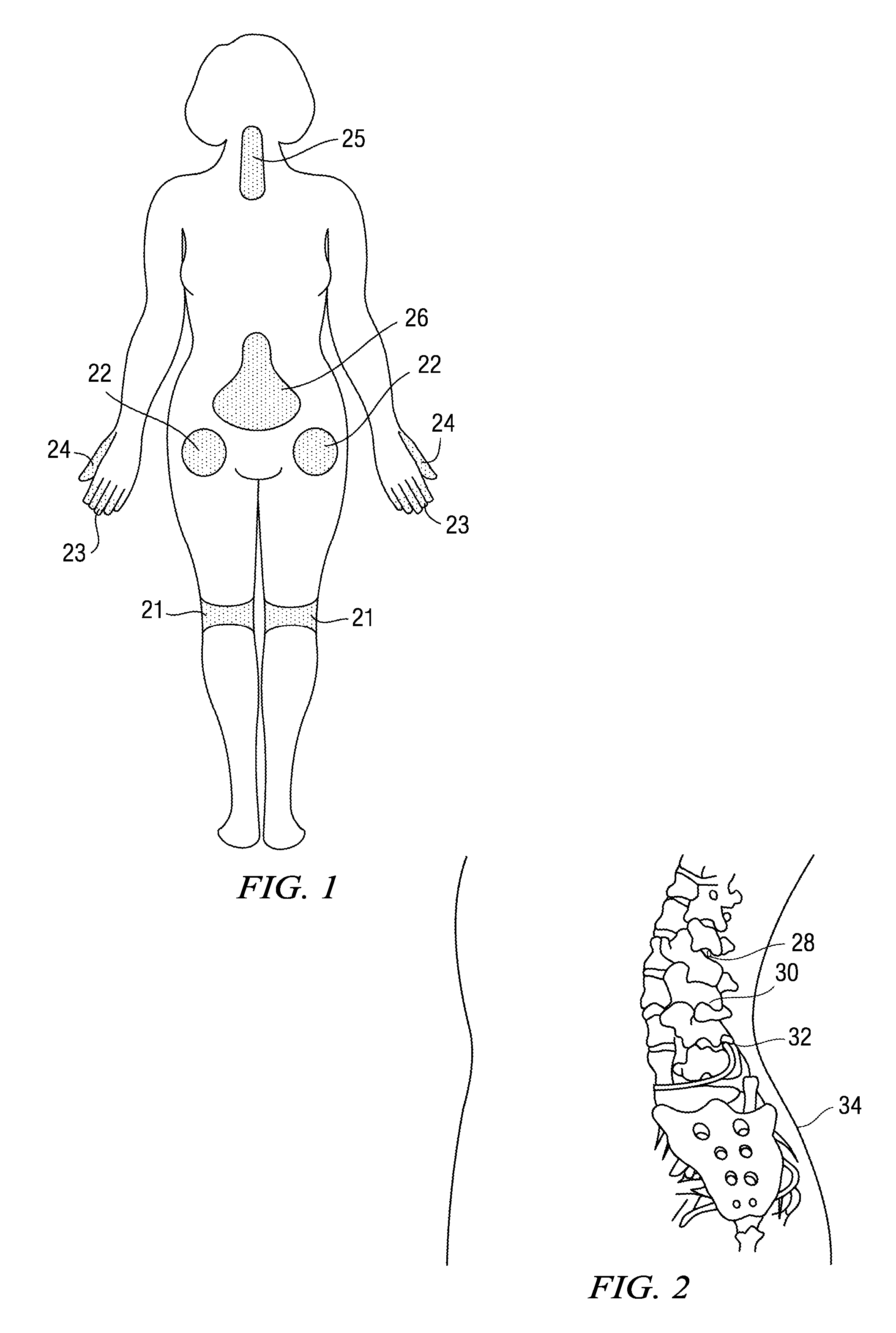
The present invention relates to delivery, including transdermal delivery, of a therapeutically effective amount of a compound useful for modulating gap junction formation and function, including an oligonucleotide for reducing gap junction formation and function, and methods and compositions for treating a subject suffering from pain associated with a disease, disorder or condition and associated with pain, including but not limited to muscle pain, ligament pain, tendon pain, joint pain and post-operative pain.
Owner:DUFT BRADFORD J +1
Long-acting polymeric delivery systems
ActiveUS20170304455A1Enhance the imageEffective pain reliefPowder deliveryPharmaceutical non-active ingredientsAnesthetic AgentActive agent
Compositions comprised of a delivery vehicle or delivery system and an active agent dispersed within the delivery vehicle or system, wherein the delivery vehicle or system contains a polyorthoester polymer and a polar aprotic solvent. Also disclosed are low viscosity delivery systems for administration of active agents. The low viscosity delivery systems have a polyorthoester polymer, a polar aprotic solvent and a solvent containing a triglyceride viscosity reducing agent. Compositions described include an amide- or anilide-type local anesthetic of the “caine” classification, and a non-steroidal anti-inflammatory drug (NSAID), along with related methods, e.g., for treatment of post-operative pain or for prophylactic treatment of pain. The compositions are suitable for delivery via, e.g., direct application and instillation, intradermal injection, subcutaneous injection, and nerve block (perineural).
Owner:HERON THERAPEUTICS
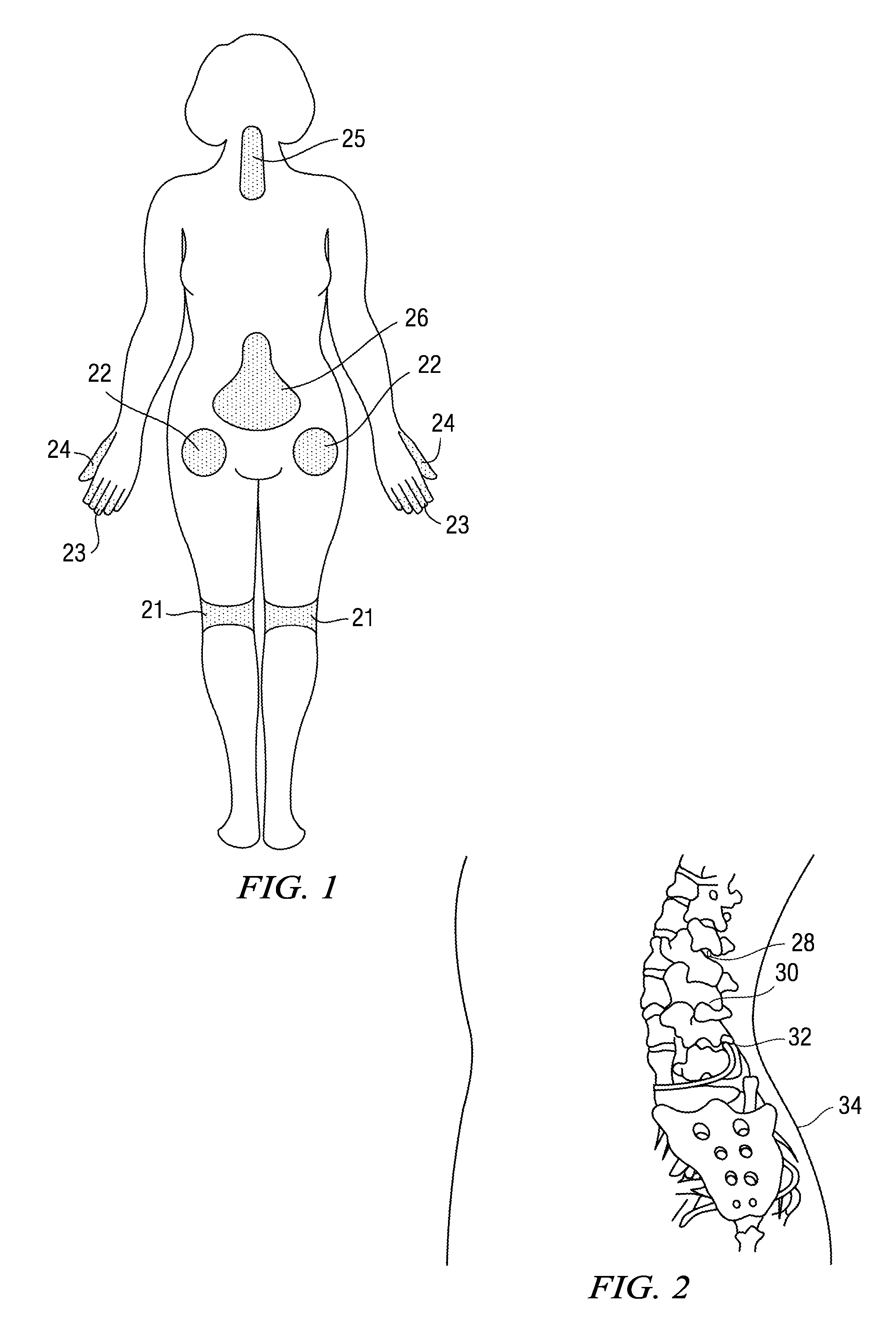
Shelf stable pharmaceutical depot
A pharmaceutical depot includes a biodegradable polymer having a glass transition temperature of 20 degrees centigrade or less and at least 25% wt solid particles suspended in the biodegradable polymer. The pharmaceutical depot also includes a post-operative pain relieving therapeutic agent.
Owner:COMPANION SPINE LLC
Methods for preparing benzo ring derivative with benzylic quaternary carbon center and ethazocine hydrobromide
InactiveCN108530241ALow priceRich varietyGroup 4/14 element organic compoundsCarbamic acid derivatives preparationHydrobromideTetralin
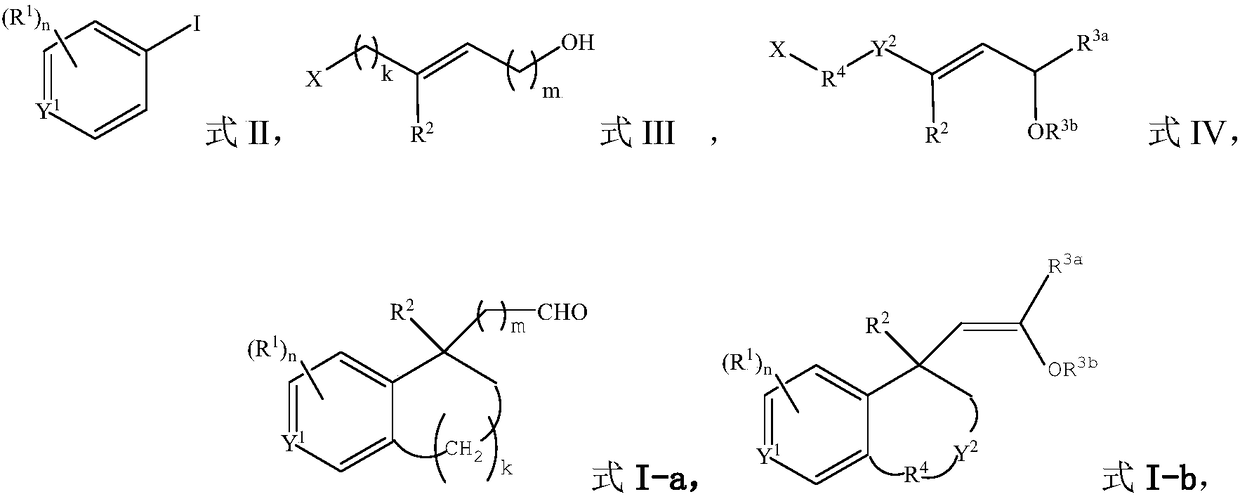
The invention provides methods for preparing a benzo ring derivative with a benzylic quaternary carbon center and ethazocine hydrobromide. An aryl iodide and an alkylating agent as starting materialsare stirred for reaction in an organic solvent at 30 DEG C to 120 DEG C under the action of a palladium catalyst, a phosphine ligand, a norbornene derivative, and a base to obtain the benzo ring derivative with the benzylic quaternary carbon center. The raw materials used in the method are cheap and easy to obtain, reaction conditions are mild, substrate universality is good, yield is high, and the preparation process is simple. Meanwhile, the present invention also provides a method for synthesizing an ethazocine hydrobromide compound based on a 1,2,3,4-tetrahydronaphthalene compound having abenzylic all-carbon quaternary carbon center synthesized by the method, the drug ethazocine hydrobromide for treatment of cancer pain and post-operative pain can be synthesized only by four steps. The method has short synthetic steps, simple operation and high total yield.
Owner:WUHAN UNIV
Compositions and method for the reduction of post-operative pain
ActiveUS8603528B2Flexibility and variation availabilityExtended elution timePowder deliveryPharmaceutical non-active ingredientsCarboxylic acidKetone
At least three component, body-implantable, absorbable, biocompatible, putty, and non-putty pain-relieving compositions for use in surgery comprising in intimate admixture: an analgesic having local pain-relieving activity for internal relief of pain, a finely powdered bulking material, preferably less than 50 microns, e.g. the metal salts of fatty acid, hydroxyapatite, DBM, polyglycolide, polylactide, polycaprolactones, absorbable glasses, gelatin, collagens, mono, and polysaccharides starches.An organic liquid capable of solubilizing, dispensing or suspending the analgesic, such as esters of monohydric alcohols with aliphatic monocarboxylic acids; C2-C18 monohydric alcohols with polycarboxylic acids; C8-C30 monohydric alcohols; tocopherol and esters thereof with mono or polycarboxylic acids; free carboxylic acids such as oleic, capric, and lauric; dialkyl ethers and ketones; polyhydroxy compounds and esters and ethers thereof; random or block copolymers of ethylene oxide and propylene oxide.
Owner:ORTHOCON INC
Disposable tissue retraction clamp
The object of the present invention is to disclose a disposable tissue retractor clip, which includes a pair of tissue clips, a tubular shell and a handle, the tubular shell is connected to the handle, and a control is arranged on the handle The tissue clip is a driving mechanism that moves back and forth in the tubular housing. A hook is connected to the front end of the driving mechanism. The hook can protrude out of the tubular housing and hook the tail end of the tissue clip. The chuck is in a clamped state under the action of its own elasticity; compared with the existing technology, it can effectively retract and fix human tissue without occupying the minimally invasive channel, and does not need to occupy the doctor's hands, reducing the minimally invasive channel quantity to reduce postoperative pain of patients, simple in structure, very convenient, and realize the purpose of the present invention.
Owner:微至(苏州)医疗科技有限公司
Simplified resiniferatoxin analogues as vanilloid receptor agonist showing excellent analgesic activity and the pharmaceutical compositions containing the same
InactiveUS6872748B2Relieve painEffectively alleviatedBiocideOrganic compound preparationIrritationJoint arthralgia
The present invention is related to new vanilloid analogues containing resiniferatoxin pharmacophores, pharmaceutical compositions that have such analogues, and their uses as vanilloid receptor agonists and potent analgesics. The present invention provides a pharmaceutical composition for preventing, alleviating or treating pain, acute pain, chronic pain, neuropathic pain, post-operative pain, migraine, arthralgia, neutopathies, nerve injury, diabetic neuropathy, neurodegeneration, neurotic skin disorder, stroke, urinary bladder hypersensitiveness, irritable bowel syndrome, a respiratory disorder such as asthma or chronic obstructive pulmonary disease, irritation of skin, eye or mucous membrane, fervescence, stomach-duodenal ulcer, inflammatory bowel disease, inflammatory disease or urgent urinary incontinence.
Owner:MEDIFRON DBT CO LTD
Use of reboxetine for the treatment of pain
InactiveCN101208095AImprove mental actionOrganic active ingredientsNervous disorderVisceral painTreatment pain
Use of (S,S)- or racemic reboxetine or a pharmaceutically acceptable salt thereof in the manufacture of a medicament for the treatment of a pain condition selected from neuropathic pain, nociceptive pain, cancer pain, back pain, inflammatory pain, musculo-skeletal disorders, visceral pain, pain from strains / sprains, post-operative pain, posttraumatic pain, burns, renal colic, acute pain, central nervous system trauma, head pain, and orofacial pain, is disclosed. Use of (S,S)-reboxetine or a pharmaceutically acceptable salt thereof in the manufacture of a medicament for the treatment of pain in a patient refractory to an alpha-2-delta ligand, and use of (S,S)- or racemic reboxetine or a pharmaceutically acceptable salt thereof in the manufacture of a medicament for use as a mental performance or mood enhancer, are also disclosed.
Owner:PFIZER INC
Shelf stable pharmaceutical depot
A pharmaceutical depot includes a biodegradable polymer having a glass transition temperature of 20 degrees centigrade or less and at least 25% wt solid particles suspended in the biodegradable polymer. The pharmaceutical depot also includes a post-operative pain relieving therapeutic agent.
Owner:COMPANION SPINE LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com