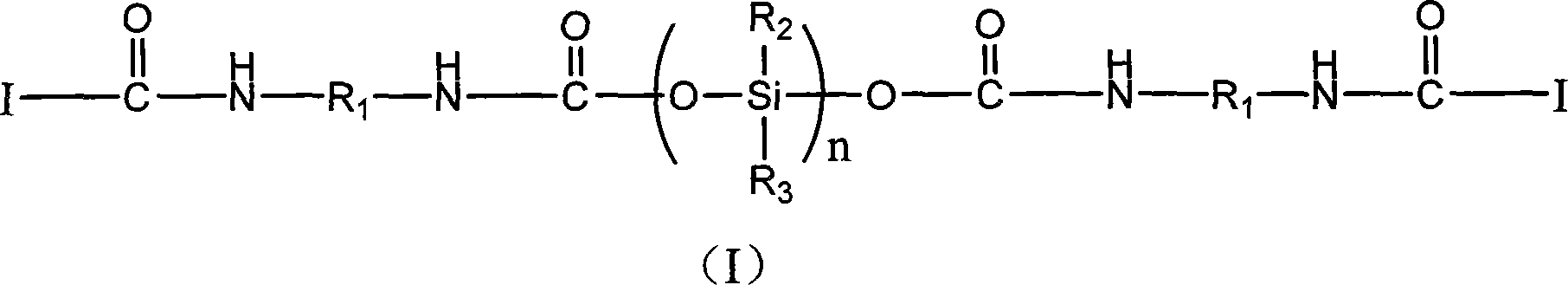
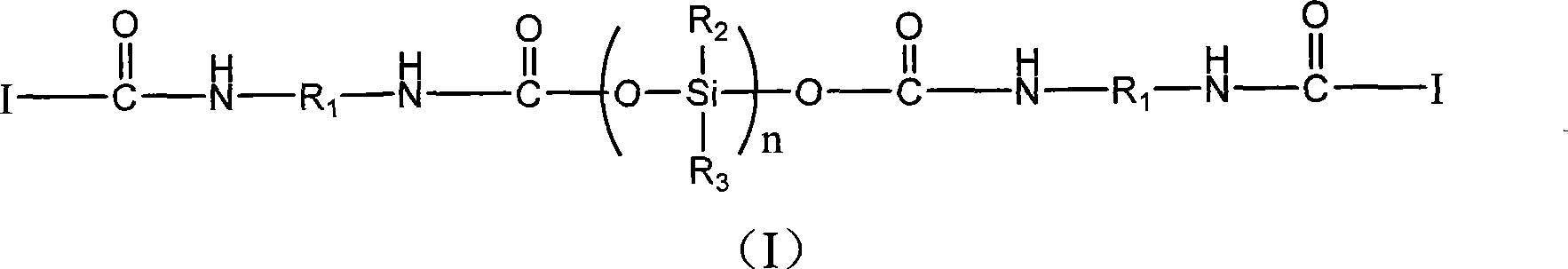Silicon containing macromolecule photo-initiation agent, synthesis and uses thereof
A technology of photoinitiators and macromolecules, applied in chemical instruments and methods, compounds of group 4/14 elements of the periodic table, organic chemistry, etc., can solve problems such as increased hazards and enhanced concentration of photolyzed products of photoinitiators , to achieve a good eliciting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of 1173-IPDI-Tego 2311 macromolecular photoinitiator
[0031] Add 17.76g (0.08mol) of IPDI (isophorone diisocyanate) and 400ml of ethyl acetate into a three-necked flask, start stirring and heating, when the temperature rises to 60°C, add 0.06g of catalyst dibutyltin dilaurate , and start to drop 13.36g (0.08mol) 1173 (hydroxyl dimethyl acetophenone), three hours later, take a sample, measure the isocyanic acid value, after reaching half of the initial value, stop the reaction, the ethyl acetate is rotated After removal from the evaporator, 1173-IPDI was obtained.
[0032] Add 50g (0.04mol) of Tego 2311 (DEGUSSA company) into a 1000ml three-neck flask, start magnetic stirring, and start heating. When the temperature rises to 75°C, start to drop 1173-IPDI15.56g ( 0.04mol), after six hours of reaction, the target product was obtained after the isocyanate group was determined to be completely reacted by infrared spectroscopy.
[0033] The product is identified ...
Embodiment 2
[0035] Synthesis of 184-TDI-Tego 2311 macromolecular photoinitiator
[0036] Add 13.92g (0.08mol) of TDI (toluene diisocyanate) and 350ml of toluene into a three-necked flask, start stirring and start heating, when the temperature rises to 60°C, add 0.06g of catalyst dibutyltin dilaurate, and start to drop 16.32g (0.08mol) 184 (1-benzoylcyclohexyl-1-alcohol), after three hours, take a sample, measure the isocyanic acid value, after reaching half of the initial value, after the solvent is removed by a rotary evaporator, obtain 184-TDI.
[0037] Add 50g (0.04mol) of Tego 2311 into a 1000ml three-necked flask, start magnetic stirring, and start heating. When the temperature rises to 75°C, start to add dropwise the
[0038] 184-TDI 15.12g (0.04mol), after six hours of reaction, the target product was obtained after the complete reaction of the isocyanate group was confirmed by infrared spectroscopy.
[0039] The product is identified by infrared spectrum and ultraviolet spectrum...
Embodiment 3
[0041] Synthesis of Benzophenone-HDI-550 Macromolecular Photoinitiator
[0042] Add 13.44g (0.08mol) of HDI (hexamethylene diisocyanate) and 400ml of tetrahydrofuran into a three-necked flask, start stirring and start heating, when the temperature rises to 60°C, add 0.06g of catalyst dibutyltin dilaurate, and Start to add 15.84g (0.08mol) of hydroxybenzophenone dropwise. After three hours, take a sample and measure the isocyanate value. After reaching half of the initial value, stop the reaction, and remove the solvent by a rotary evaporator to obtain 184-HDI.
[0043]Add 50g (0.04mol) of polydimethylsilane 550 (ACROS company) into a 1000ml three-necked flask, start magnetic stirring, and start heating. When the temperature rises to 60°C, start to drop the diphenyl Ketone-HDI 14.64g (0.04mol). After six hours of reaction, the target product was obtained after the isocyanate group was determined to be completely reacted by infrared spectroscopy.
[0044] The product is identif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com