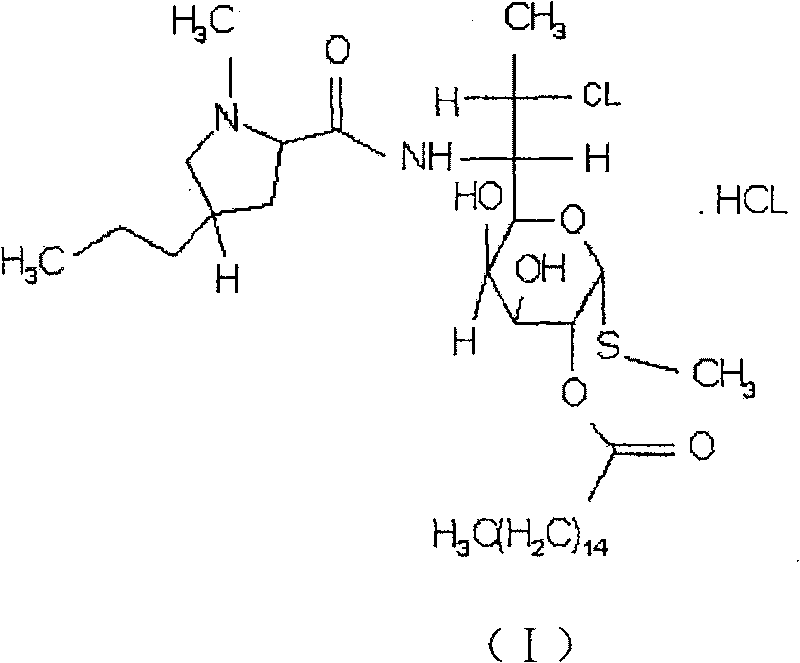
Antibacterium compounds containing clindamycin hydrochloride
A technology of clindamycin hydrochloride and antibacterial composition, which is applied in the field of antibacterial compositions containing clindamycin hydrochloride palmitate, can solve the problems of no antibacterial activity, limited antibacterial application, etc., and achieves widening antibacterial scope and application. promising effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 ointment preparation
[0019] The components and contents are as follows:
[0020] Clindamycin Palmitate Hydrochloride 1g
[0021] Manidipine 4g
[0022] Vaseline 80g
[0023] Liquid paraffin 9g
[0024] Lanolin 8g
[0025] Pure water 1.5g
[0026] According to the above components and contents, add epinastine hydrochloride and prednisolone into pure water to dissolve, add vaseline-liquid paraffin-lanolin ointment base, mix evenly, separate bacteria, and pack separately to obtain the finished product.
Embodiment 2
[0027] Embodiment 2 capsule preparation
[0028] The components and contents are as follows:
[0029] Clindamycin Palmitate Hydrochloride 1g
[0030] Manidipine 2.5g
[0031] Cyclodextrin 5g
[0032] Microcrystalline Cellulose 60g
[0033] Calcium hydrogen phosphate 20g
[0034] Carboxymethyl Starch Sodium 25g
[0035] Stevia 25g
[0036] Magnesium Stearate 5g
[0037] Micronized silica gel 10g
[0038] Starch lactose 100g
[0039] The above-mentioned various raw materials are dried at low temperature, pulverized, passed through a 100-mesh fine sieve, mixed uniformly, made into a powder, and uniformly granulated by a granulator, and filled into a hollow capsule with a capsule filler after sterilization to obtain a capsule. Capsules contain 0.35 g of granules.
Embodiment 3
[0040] Embodiment 3 antibacterial experiment
[0041] 1 g of clindamycin palmitate hydrochloride and 2.5 g of manidipine are prepared into a combined preparation, and the production process of conventional freeze-dried powder injection is carried out.
[0042] The combined preparation of the invention has been proved by in vitro tests that its antibacterial activity is stronger than that of clindamycin palmitate hydrochloride, and its antibacterial range is wider.
[0043] The antibacterial effect (MIC of table 1 combination preparation and clindamycin hydrochloride palmitate 90 mg / L)
[0044] bacteria
combination preparation
Clindamycin Palmitate Hydrochloride
Staphylococcus aureus
1.2
2.5
Staphylococcus epidermidis
10
18
Streptococcus pneumoniae
5
8
Escherichia coli
2
-
Klebsiella
0.1
-
Pseudomonas aeruginosa
0.2
-
Propionibacterium
6
5
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com