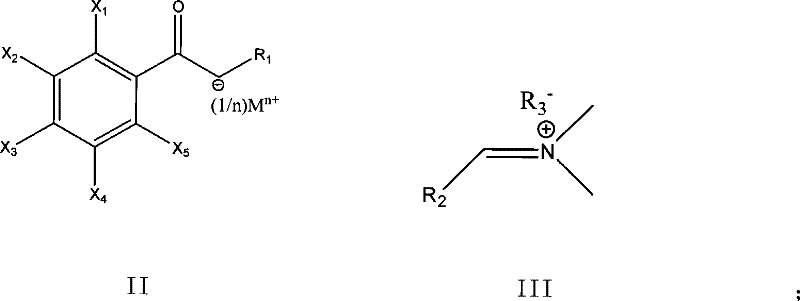
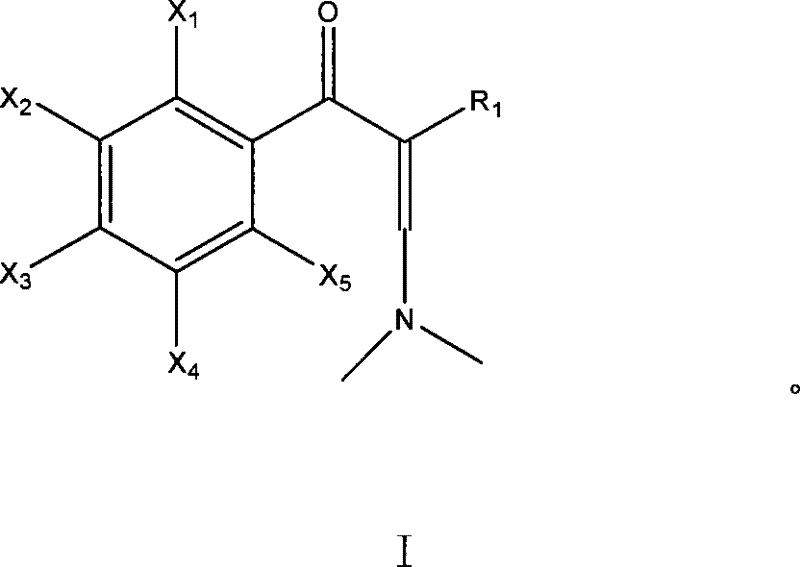
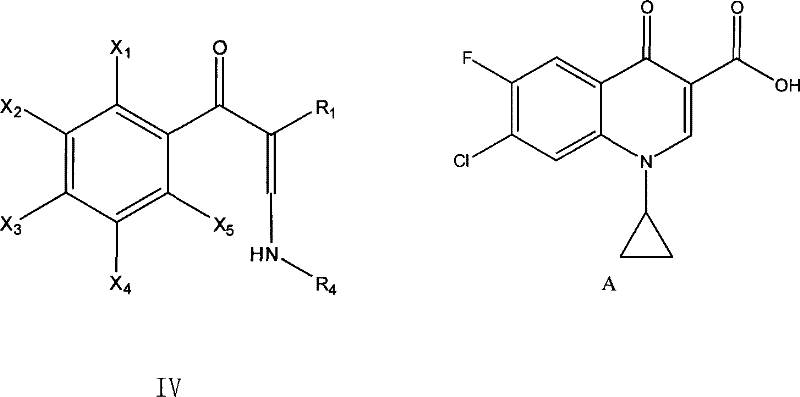
Method for preparing enamine derivates
A technology for derivatives and enamines, applied in the field of preparation of enamine derivatives, can solve the problems of large consumption of triethyl orthoformate and acetic anhydride, unsuitable for large-scale production, trivial preparation of DMFA, etc., and achieves low cost and high reaction efficiency. Mild conditions and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1, the preparation of imide salt (Methoxymethylen) dimethylammonium-methylsulfat (molecular formula such as V):
[0049]
[0050] Add DMF (37.0g, 0.51mol) into a 250ml three-necked flask, stir and raise the temperature to 55°C, slowly add dimethyl sulfate (63.1g, 0.50mol) dropwise, maintain the temperature in the flask at 50-60°C, and drop for about 60min complete. After dropping, raise the temperature to 75°C and keep it warm for 3 hours. After cooling, the imide salt can be obtained.
Embodiment 2
[0051]The preparation of embodiment 2, 3-(S-hydroxypropyl-2-amine)-2-(2,3,4,5-benzoyl) ethyl acrylate, the following steps are carried out successively:
[0052] 1. Throw 9.7g of sodium hydride and 200ml of toluene into a 500ml four-neck flask, and add dropwise 2,3,4,5-tetrafluorobenzoyl ethyl acetate solution (40g / 69ml of toluene, that is, 69ml Add 40g of ethyl 2,3,4,5-tetrafluorobenzoylacetate to toluene), drop it in about 2 to 3 hours, and react at 0°C for 8 hours to obtain 2,3,4,5- Toluene solution of ethyl tetrafluorobenzoyl acetate sodium salt.
[0053] 2. At about -5°C, add 61.5 g of the imide salt obtained in Example 1 dropwise to the toluene solution of the above-mentioned 2,3,4,5-tetrafluorobenzoyl ethyl acetate sodium salt (the molar ratio is 1: 2.04 ), the drop was completed in about 1.5 hours, and after the drop was completed, the reaction was incubated at -5°C for 15 hours, and the end point of the reaction was detected by spotting the plate. After the reaction...
Embodiment 3
[0055] Example 3, the preparation of 3-cyclopropylamino-2-(2,4-dichloro-5-fluorobenzoyl)acrylic acid acetonitrile, the following steps were carried out in sequence:
[0056] 1. Throw 6.5g of sodium hydride and 200ml of toluene into a 500ml four-neck flask. Control the temperature at about 0°C and add 2,4-dichloro-5-fluorobenzoyl acetonitrile solution (37g / 70ml toluene) dropwise, drop it in about 2 to 3 hours, and keep the reaction at the above temperature for 8 hours to obtain 2 , 4-Dichloro-5-fluorobenzoylacetonitrile sodium salt in toluene.
[0057] 2. Add dropwise 40 g of the imide salt (2,4-dichloro-5-fluoro The molar ratio of benzoyl acetonitrile to imide salt is 1:1.26), and the dripping is finished in about 2 hours; after the dripping, the temperature is kept at this temperature for 5 hours, and the temperature is raised to 40°C for 10 minutes after the warming, suction filtration, and the filtrate is recovered under reduced pressure To dryness, 3-dimethylamino-2-(2,4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com