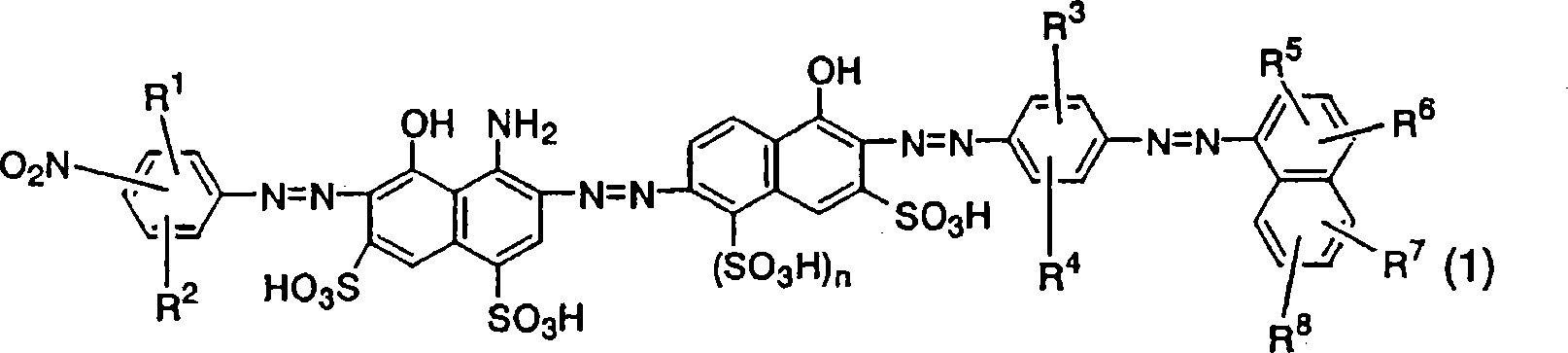
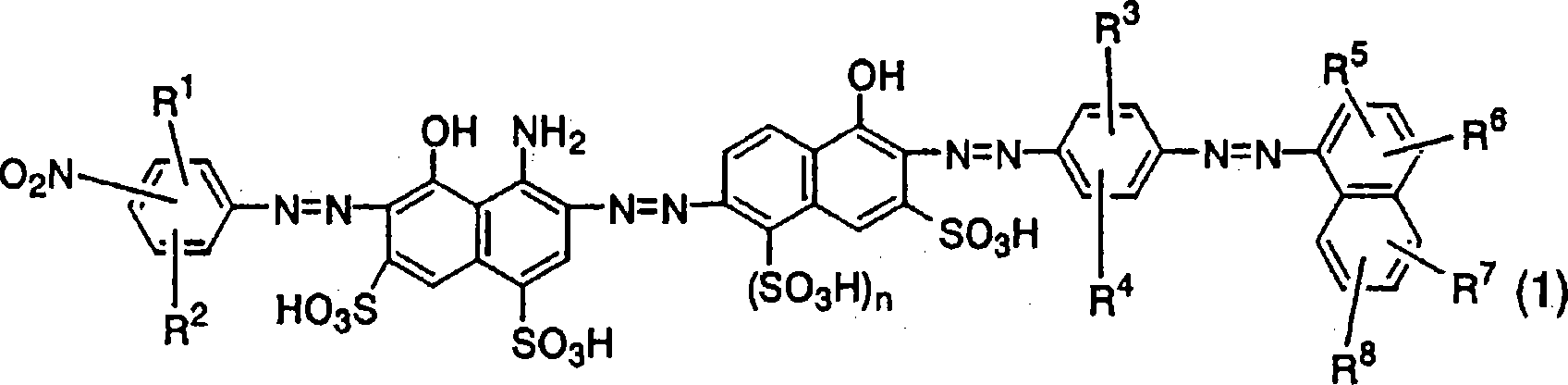
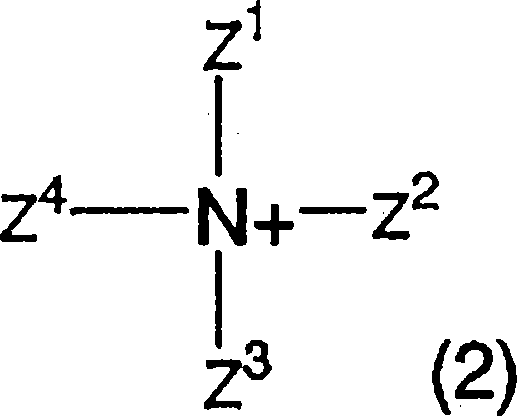Azo compound, ink composition, recording method, and colored object
An azo compound and azo-based technology, used in the fields of azo compounds, ink compositions, recording and coloring bodies, can solve the problems of insufficient ozone resistance and market requirements, and achieve stable ejection. Excellent properties, excellent storage stability, excellent water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] (1) Take 6.4 parts of 2-amino-5-naphthol-1,7-disulfonic acid and 4.1 parts of p-toluenesulfonyl chloride in water, react for 1 hour at a pH of 8.0 to 8.5 at a temperature of 70°C, Adjust the reaction solution to be acidic for salting out, and filter the precipitate to obtain the compound of formula (12). 8.8 parts of the obtained compound were dissolved in 90 parts of water, and dissolved while adjusting the pH to 6.0 to 8.0 with sodium carbonate. After adding 6.8 parts of 35% hydrochloric acid to the obtained solution, after cooling to 0 to 5°C, 3.6 parts of 40% sodium nitrite aqueous solution were added to diazotize the compound of formula (12).
[0120]
[0121] To this diazo suspension, add a solution obtained by suspending 5.8 parts of 4-amino-5-hydroxynaphthalene-1,7-disulfonic acid in 60 parts of water, and then place the resulting suspension at 10 to 20°C It was stirred for 4 hours while maintaining its pH at 2.4 to 2.8 with sodium carbonate. Then, adjust t...
Embodiment 2
[0133] (A) Preparation of ink
[0134] The black ink composition of the present invention was prepared by mixing the following ingredients, and then filtered through a 0.45 μm membrane filter to remove impurities therein.
[0135] In addition, ion-exchanged water was used for water. When preparing the ink, adjust the pH of the ink to 7 to 9 with ammonium hydroxide, and then add ion-exchanged water to make the total amount 100 parts.
[0136] table 3
[0137] 5.0 parts of the compound obtained in the above-mentioned Example 1
[0138] Glycerol 5.0 parts
[0139] Urea 5.0 parts
[0140] N-methyl-2-pyrrolidone 4.0 parts
[0141] 3.0 parts of isopropanol
[0142] Butyl Carbitol 2.0 parts
[0143] Surfactant (Surfynol 105 manufactured by Nissin Chemical Co., Ltd.) 0.1 part
[0144] Water+ammonium hydroxide 75.9 parts
[0145] Total 100.0 copies
[0146] The compounds prepared in the above-mentioned Example 1 in Table 3 represent the compounds of formula (17). The water-bas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| survival rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com