Process for the preparation of benzotriazepine derivatives
A compound, CH2 technology, applied in the direction of active ingredients of heterocyclic compounds, drug combination, organic chemistry, etc., can solve the problems of high price, slow reaction steps, disadvantages, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
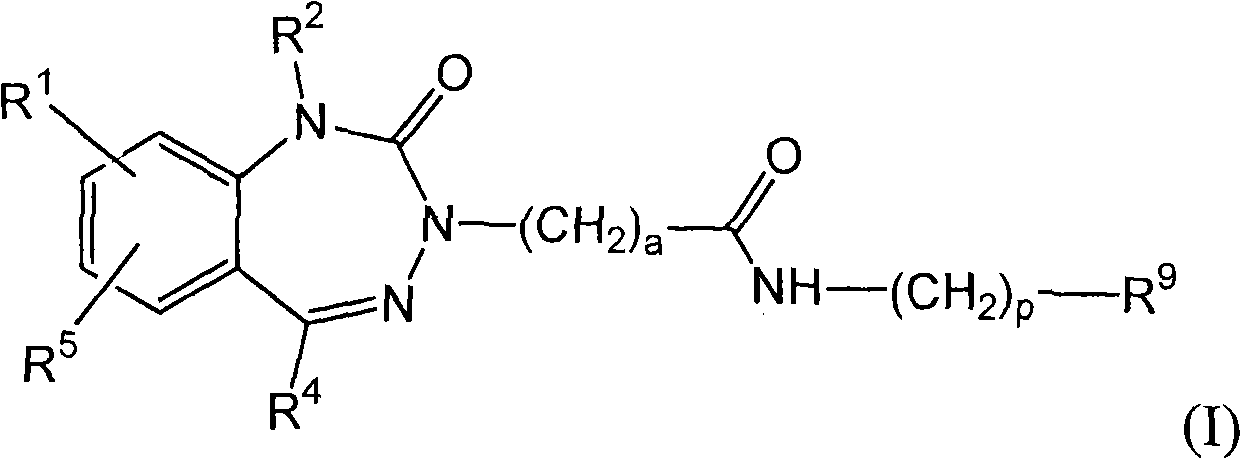
[0079] The present invention relates to the preparation method of following formula (X) compound:
[0080]
[0081] where R 1 , R 5 , R 2 , R 4 , a and R 9 as defined herein. The compound of formula (I) is benzotriazepine Derivatives for use in the treatment of diseases mediated by gastrin and cholecystokinin receptors, as disclosed in PCT publication WO 2003 / 041714.
[0082] The term "hydrocarbyl" as used herein refers to a monovalent group consisting of carbon and hydrogen. Hydrocarbyl groups include alkyl, alkenyl and alkynyl groups (in both straight and branched chain forms), cycloalkyl groups (including polycyclic alkyl groups such as bicyclooctyl and adamantyl), cycloalkenyl and aryl groups and the aforementioned Combinations of groups such as alkylcycloalkyl, alkylpolycycloalkyl, alkylaryl, alkenylaryl, alkynylaryl, cycloalkylaryl, and cycloalkenylaryl.
[0083] Where reference is made to a carbon atom of a hydrocarbyl group replaced by a N, O or S atom, it ...
Embodiment 1
[0198] [3-(2-Cyclohexanecarbonyl-phenyl)-1-amino-ureido]-ethyl acetate
[0199]
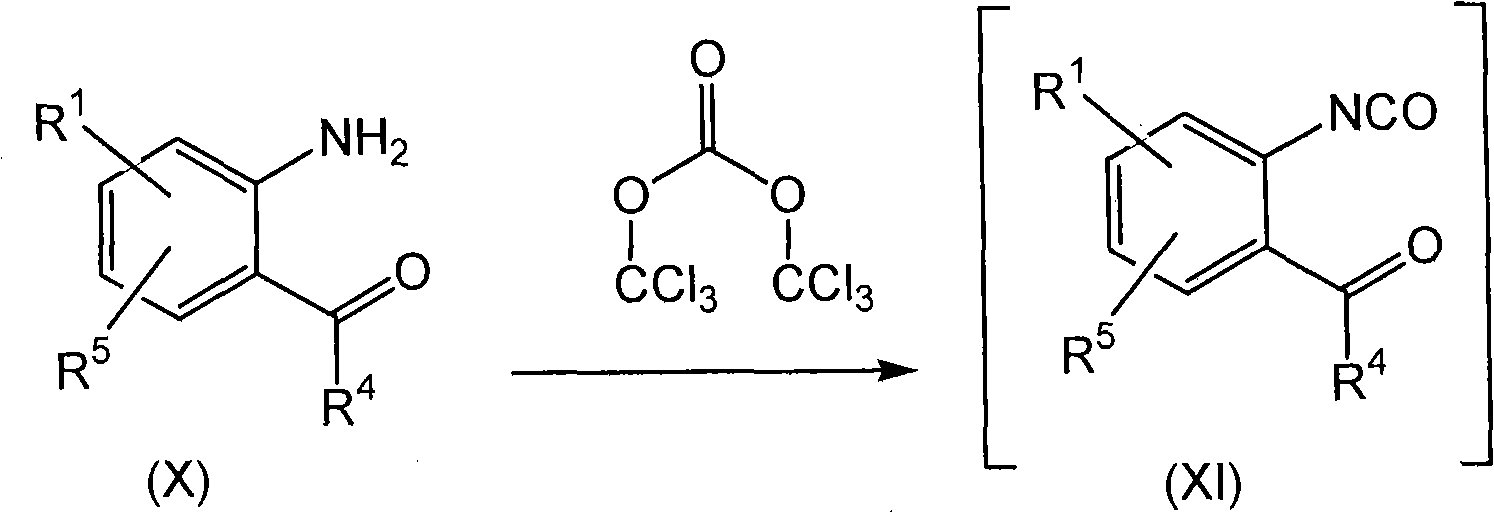
[0200] To a solution of (2-amino-phenyl)-cyclohexyl-methanone (1.0 g, 5 mmol) in DCM (15 ml) was added triethylamine (3.6 ml) and cooled to -50°C. At this temperature, a solution of triphosgene (also known as bis(trichloromethyl carbonate)) (0.5 g) in DCM (5 ml) was added all at once, and after the temperature was observed to rise to -10 ° C, the resulting solution was heated at -10 ° C and Matured at -20°C for about 15 minutes, then slowly transferred to a flask via a syringe containing ethyl hydrazinoacetate hydrochloride (0.5g, 3.2mmol) and triethylamine (1ml) with DCM (10ml) syrup. A rise in temperature from 23°C to 26°C was noted during the addition. After the reaction mixture was aged at ambient temperature for 2 hours, the insoluble material (triethylamine hydrochloride) was removed by filtration. The filtrate was concentrated in vacuo and the resulting residue was dissolved in iso...
Embodiment 2
[0205] (5-cyclohexyl-2-oxo-1,2-dihydro-benzo[e][1,2,4]triazepine -3-yl)-ethyl acetate
[0206]
[0207] Dissolve ethyl [1-[[[2-(cyclohexylcarbonyl)phenyl]amino]carbonyl]hydrazino]-acetate (20 mg) in TFA (0.2 ml) and mature for about 15 minutes. At this point HPLC analysis showed the disappearance of the signal corresponding to starting material at 10.5 minutes and the appearance of a signal corresponding to product at 11.01 minutes. The material was characterized by comparison with the signal from the HPLC trace of real samples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com