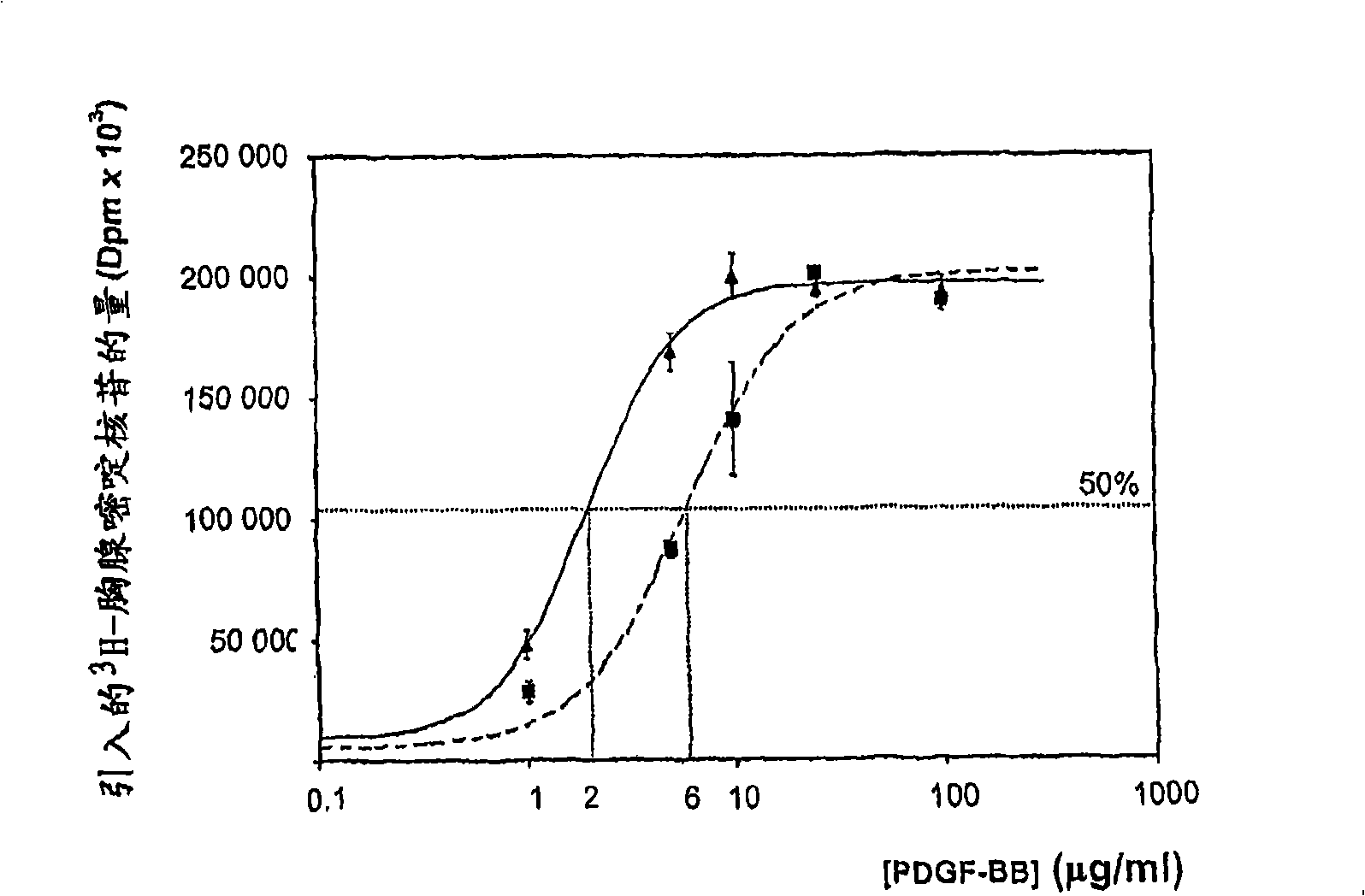
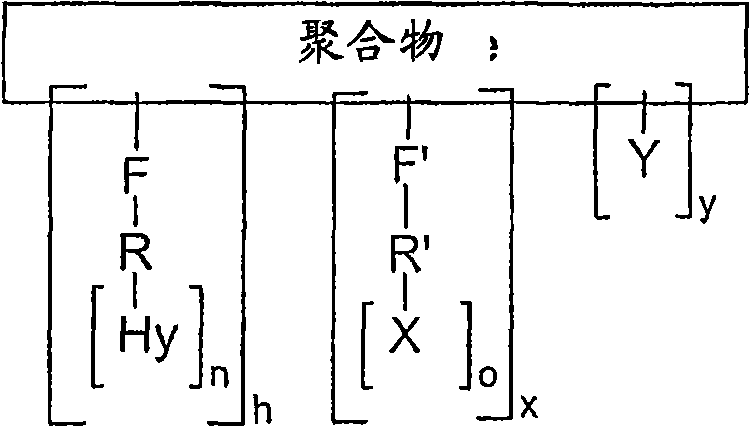
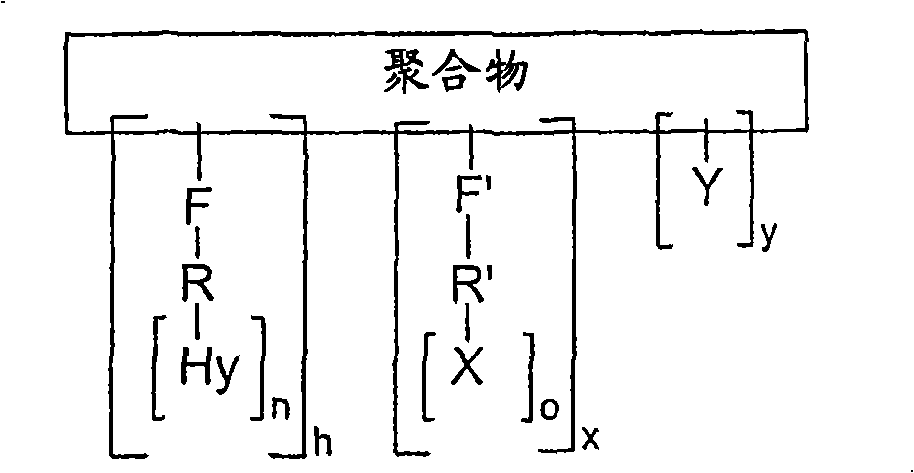
PDGF amphiphilic polymer complex
A technology of amphiphilic polymers and complexes, which can be used in drug combinations, medical preparations with non-active ingredients, digestive systems, etc., and can solve problems such as inability to stabilize protease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Example 1: PDGF-BB / DMCBSu complex
[0150] Synthesis of Sulfated Carboxymethyl Dextran (DMCBSu) Modified with Benzylamine
[0151] The amphiphilic polymer is synthesized from carboxymethyl dextran with a degree of carboxymethyl substitution per sugar unit of 1.0 and an average molar mass of 40,000 g / mol. According to the traditional coupling method, benzylamine is grafted onto the acid of this polymer in the presence of water-soluble carbodiimide in water. The degree of benzylamine substitution for each sugar unit is determined by 1 It was determined by H NMR to be 0.4. Then use SO 3 The / pyridine complex sulfates the polymer. The degree of sulfate substitution per sugar unit is 0.3.
[0152] Preparation of PDGF-BB / DMCBSu complex
[0153] Add 10 microliters of 0.1 mg / ml PDGF-BB solution to 90 microliters of 50 mg / ml DMCBSu solution. The PDGF-BB and DMCBSu solutions were buffered at pH 7.4 and 300 mOsm. The solution was stirred gently at ambient temperature for 2 hours and t...
Embodiment 2
[0170] Example 2: PDGF-BB / DMCTrpOMe complex
[0171] Synthesis of carboxymethyl dextran (DMCTrpOMe) modified with tryptophan methyl ester
[0172] The amphiphilic polymer is synthesized from carboxymethyl dextran with a degree of carboxymethyl substitution per sugar unit of 1.0 and an average molar mass of 40,000 g / mol. According to the traditional coupling method, tryptophan methyl ester is grafted onto the acid of this polymer in the presence of water-soluble carbodiimide in water. The degree of tryptophan substitution of each sugar unit is determined by 1 It was determined by H NMR to be 0.3.
[0173] Preparation of PDGF-BB / DMCTrpOMe complex
[0174] Add 10 microliters of 0.1 mg / ml PDGF-BB solution to 90 microliters of 50 mg / ml DMCTrpOMe solution. The PDGF-BB and DMCTrpOMe solutions were buffered at pH 7.4 and 300 mOsm. The solution was stirred gently at ambient temperature for 2 hours and then stored at 4°C.
[0175] Verification of the formation of PDGF-BB / DMCTrpOMe complex
...
Embodiment 3
[0182] Example 3: PDGF-BB / CMCBSu complex
[0183] Synthesis of Sulfated Carboxymethyl Cellulose (CMCBSu) Modified with Benzylamine
[0184] The amphiphilic polymer is synthesized from carboxymethyl cellulose with a degree of carboxymethyl substitution per sugar unit of 1.2 and an average molar mass of 30,000 g / mol. According to the traditional coupling method, benzylamine is grafted onto the acid of this polymer in the presence of water-soluble carbodiimide in water. The degree of benzylamine substitution for each sugar unit is determined by 1 It was determined by H NMR to be 0.2. In SO 3 -Sulfation is carried out in the presence of pyridine complex, and the degree of sulfate radical substitution is 0.30.
[0185] Preparation of PDGF-BB / CMCBSu complex
[0186] Add 10 microliters of 0.1 mg / ml PDGF-BB solution to 90 microliters of 50 mg / ml CMCB solution. The PDGF-BB and CMCBSu solutions were buffered at pH 7.4 and 300 mOsm. The solution was stirred gently at ambient temperature for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com