Monoanthracene derivative blue electroluminescent material, preparation and use in electroluminescent device manufacture
A technology of electroluminescent materials and anthracene derivatives, which is applied in the direction of luminescent materials, electrical solid devices, electrical components, etc., can solve the problems of blue light materials to be developed, and achieve the effect of excellent luminous performance and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
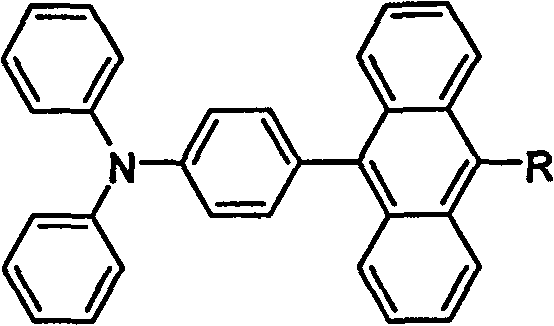
[0052] The preparation method of luminescent material of the present invention; comprises the following steps:
[0053] The first step: compound (2) is synthesized according to the following reaction formula:
[0054]
[0055] Dissolve 4-bromotriphenylamine (3.24g, 10mmol) in redistilled tetrahydrofuran (30mL), cool down to -78°C under nitrogen atmosphere, add n-butyllithium (4.8mL, 12mmol), keep the low temperature for about 1h, add iso Propoxy pinacid borate (2.45 mL, 12 mmol), warmed up to room temperature and stirred overnight. After the reaction was completed, the solvent was removed by rotary evaporation, and a white solid product was obtained by silica gel column chromatography, namely 2.2 g of 4-dianilinophenylboronic acid pinacolate of the intermediate compound (2).
[0056] The second step: compound (3) is synthesized according to the following reaction formula:
[0057]
[0058]Compound (2) (1.86g, 5mmol), 9,10-dibromoanthracene (5.04g, 15mmol), tetrahydrofu...
Embodiment 1
[0062] Embodiment 1: the synthesis of compound 2, synthetic route is as follows:
[0063]
[0064] Dissolve 9-benzanthracene (1.5g, 5.9mmol) in acetic acid (80mL), heat to reflux at 65°C under nitrogen, dissolve bromine (0.34mL, 6.5mmol) in 20mL acetic acid, and add dropwise gradually. After the bromine was added dropwise, it was lowered to room temperature, and a shiny yellow solid was obtained by filtration. The solvent was removed from the filtrate under reduced pressure to obtain a yellow solid, which was purified by recrystallization with acetic acid. The two parts of solids were combined to obtain compound (1), 1.8 g of 9-bromo-10-benzanthracene.
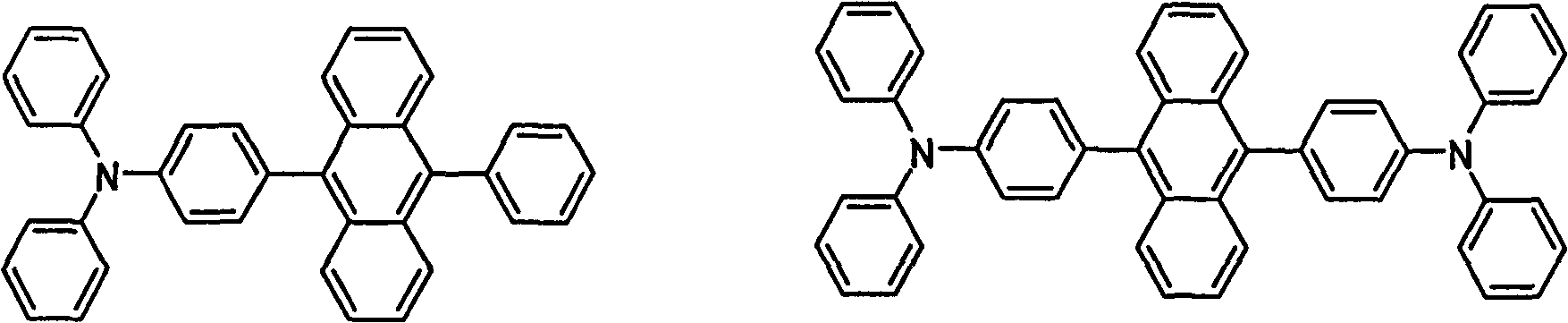
[0065] Mix compound (1) (1g, 3mmol) and compound (2) (1.655g, 3mmol) in a three-neck flask, add redistilled tetrahydrofuran (50mL), potassium carbonate solution (2.0M, 30mL), a few drops of aliquat336, catalyst Tetrakis(triphenylphosphine)palladium (100mg), heated to reflux at 100°C for 48h under nitrogen atmosphere. Afte...
Embodiment 2
[0067] Embodiment 2: the synthesis of compound 3, synthetic route is as follows:
[0068]
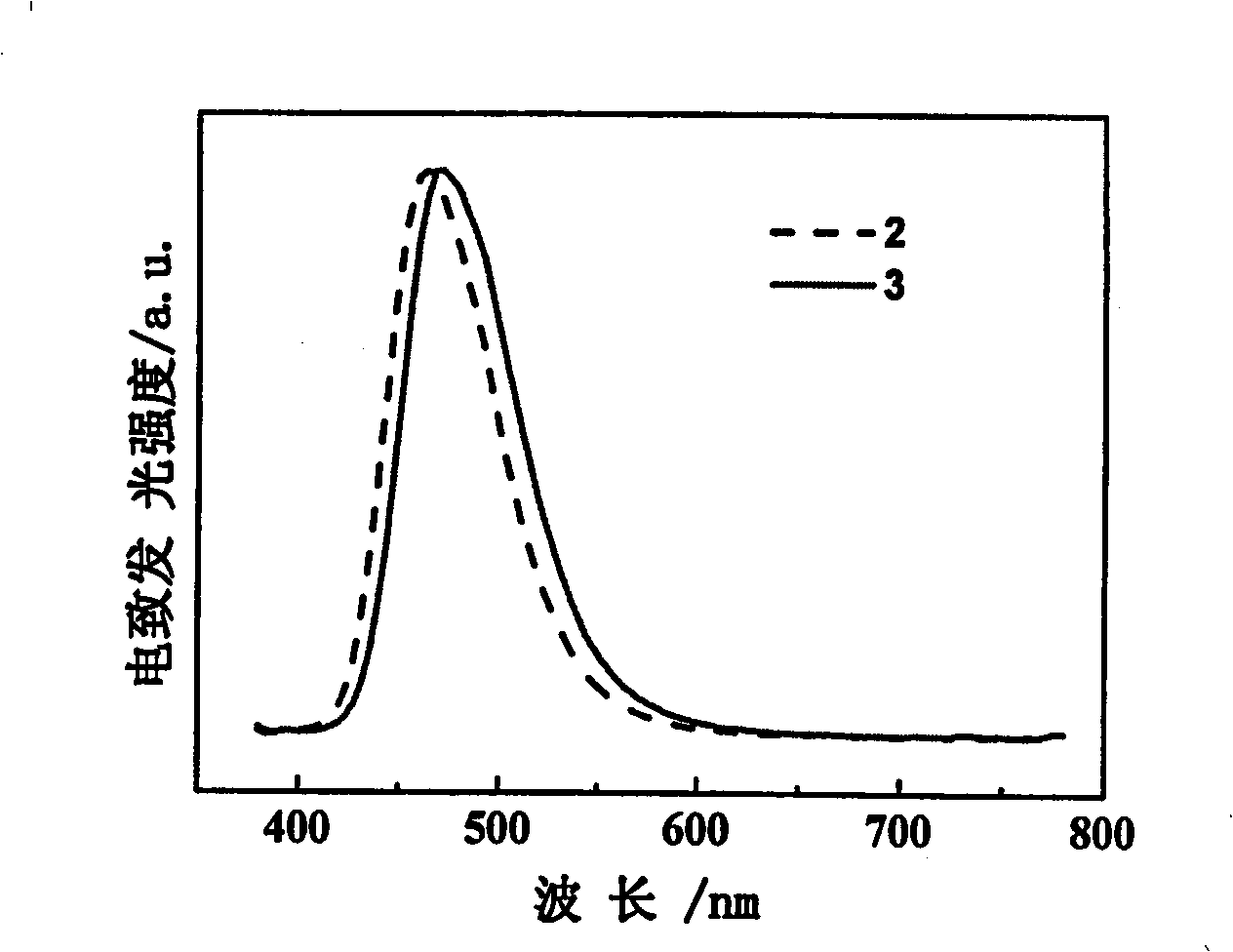
[0069] Compound (2) (3g, 5.5mmol), 9,10-dibromoanthracene (845mg, 2.5mmol), redistilled tetrahydrofuran (100mL), potassium carbonate aqueous solution (2.0M, 50mL), a few drops of aliquat336, catalyst four ( Triphenylphosphine) palladium (100 mg) was added into a three-necked flask, and heated to 100° C. under nitrogen atmosphere and heated to reflux for 48 h. After the reaction, separate the liquids, wash the aqueous phase twice with tetrahydrofuran, combine the organic phases, concentrate under reduced pressure, a precipitate precipitates out of the solution, and after filtration, a yellow-green solid 9,10-bis(4-dianilinophenyl ) anthracene (compound 3) 1.18g, yield 71.0%.
[0070] Application of the compound of general formula 1 of the present invention in the preparation of organic electroluminescent devices:
[0071] The ITO glass is washed with detergent, deionized water, acet...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap