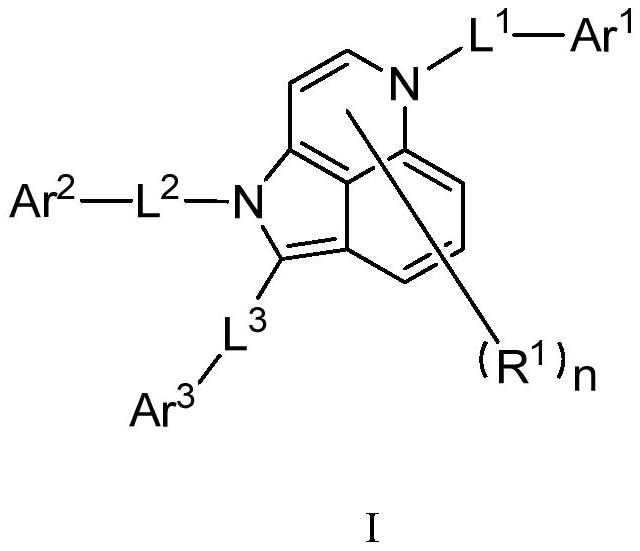
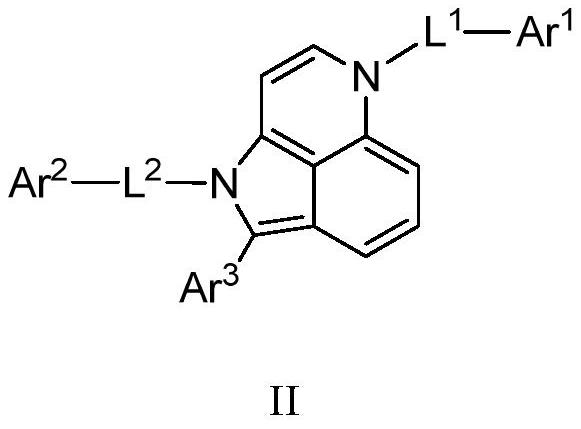
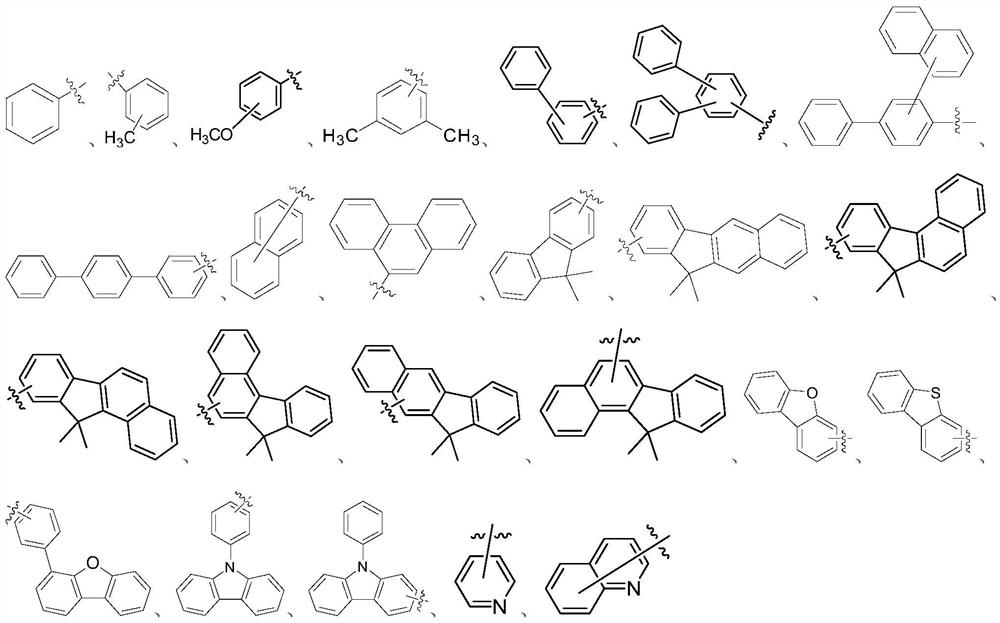
Compound, application thereof and organic light-emitting device adopting compound
A compound of general formula, selected technology, applied in the field of organic electroluminescent devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0061] Synthesis Example 1: Synthesis of Compound P1
[0062]
[0063] In a 1000ml one-port bottle, add 11.5g (50mmol) M1, 10.9g (50mmol) BOC anhydride, 20.7g (150mmol) K 2 CO 3 , 500Ml THF, stirred at room temperature for 12h, then filtered, and concentrated mother liquor M1-1.
[0064] In a 1000ml single-port bottle, add 16.5g (50mmol) M1-1, 11.6g (50mmol) 3-bromobiphenyl, 0.9g (1mmol) tris(dibenzylideneacetone) dipalladium (ie Pd 2 (dba) 3 ), 0.5mL tri-tert-butylphosphine ((t-Bu) 3P), 500ml of toluene (Toluene), 14.4g (150mmol) of sodium tert-butoxide (NaOBu-t), evacuated and changed nitrogen for 3 times, and the reaction temperature was raised to 110° C. for 5 hours. After the reaction is complete, stop the reaction. Cool to room temperature, separate the reaction solution, concentrate the organic phase, add methanol and stir for 1 h, and filter with suction to obtain light yellow powder M1-2.
[0065] In a 1000ml single-necked bottle, add 19.2g (50mmol) M1-2, 7.8...
Synthetic example 2
[0066] Synthesis Example 2: Synthesis of Compound P2
[0067]
[0068] In a 1000ml one-port bottle, add 11.5g (50mmol) M1, 10.9g (50mmol) BOC anhydride, 20.7g (150mmol) K 2 CO 3 , 500Ml THF, stirred at room temperature for 12h, then filtered, and concentrated mother liquor M1-1.
[0069] In a 1000ml single-port bottle, add 16.5g (50mmol) M1-1, 11.6g (50mmol) 3-bromobiphenyl, 0.9g (1mmol) tris(dibenzylideneacetone) dipalladium (ie Pd 2 (dba) 3 ), 0.5mL tri-tert-butylphosphine ((t-Bu) 3 P), 500ml of toluene (Toluene), 14.4g (150mmol) of sodium tert-butoxide (NaOBu-t), evacuated and changed nitrogen for 3 times, and the reaction temperature was raised to 110° C. for 5 hours. After the reaction is complete, stop the reaction. Cool to room temperature, separate the reaction solution, concentrate the organic phase, add methanol and stir for 1 h, and filter with suction to obtain light yellow powder M1-2.
[0070] In a 1000ml single-port bottle, add 19.2g (50mmol) M1-2, 1.05...
Synthetic example 3
[0071] Synthesis Example 3: Synthesis of Compound P3
[0072]
[0073] In a 1000ml one-port bottle, add 11.5g (50mmol) M1, 10.9g (50mmol) BOC anhydride, 20.7g (150mmol) K 2 CO 3 , 500Ml THF, stirred at room temperature for 12h, then filtered, and concentrated mother liquor M1-1.
[0074] In a 1000ml single-port bottle, add 16.5g (50mmol) M1-1, 11.6g (50mmol) 3-bromobiphenyl, 0.9g (1mmol) tris(dibenzylideneacetone) dipalladium (ie Pd 2 (dba) 3 ), 0.5mL tri-tert-butylphosphine ((t-Bu) 3 P), 500ml of toluene (Toluene), 14.4g (150mmol) of sodium tert-butoxide (NaOBu-t), evacuated and changed nitrogen for 3 times, and the reaction temperature was raised to 110° C. for 5 hours. After the reaction is complete, stop the reaction. Cool to room temperature, separate the reaction solution, concentrate the organic phase, add methanol and stir for 1 h, and filter with suction to obtain light yellow powder M1-2.
[0075] In a 1000ml one-port bottle, add 19.2g (50mmol) M1-2, 10.5g (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com