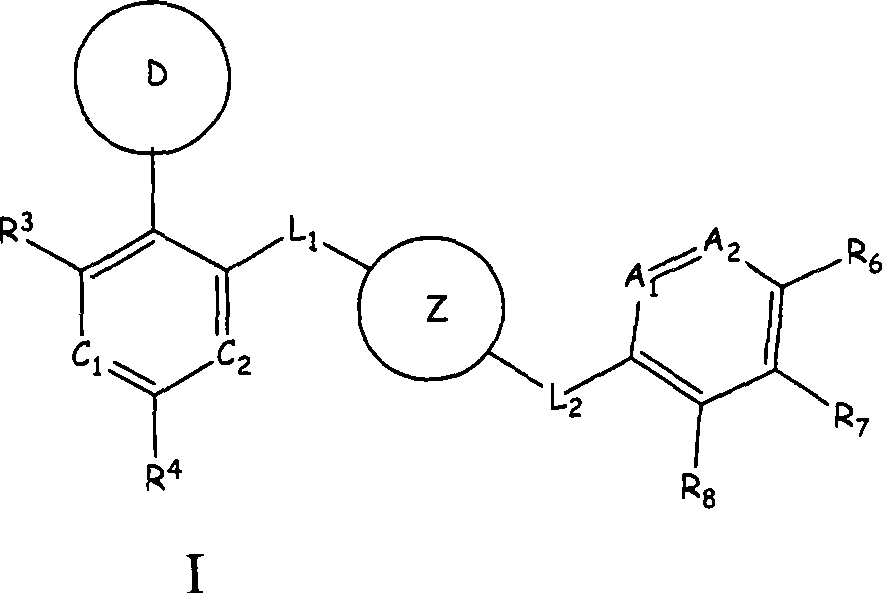
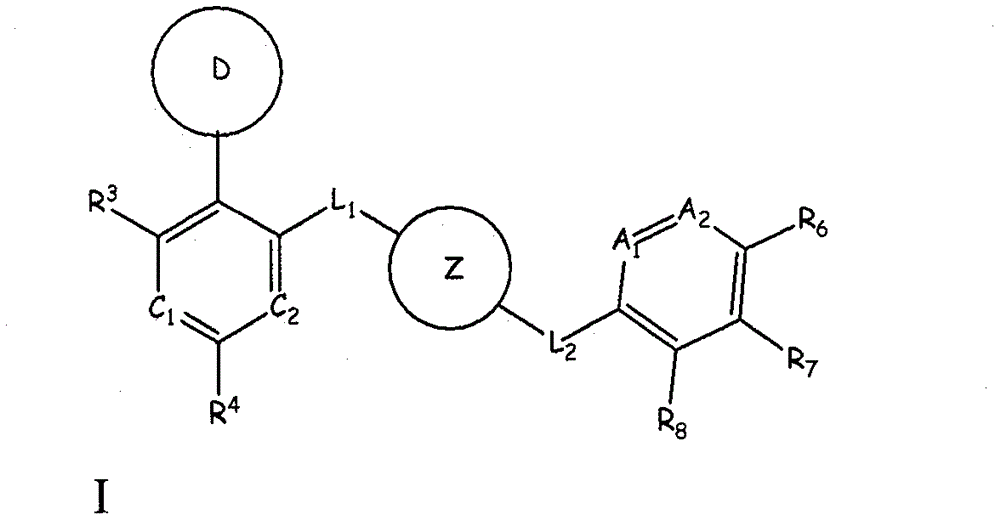
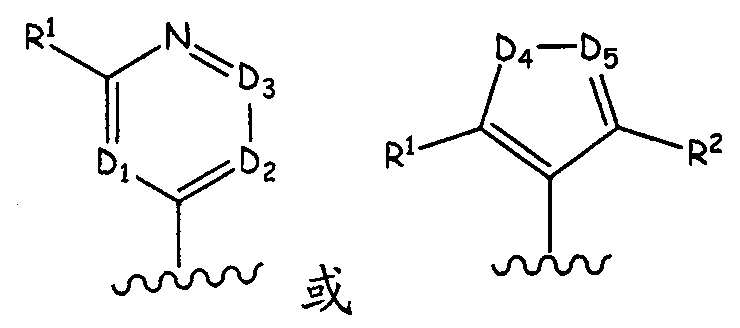
Aurora kinase modulators and method of use
A technology of alkyl and compounds, used in the field of compounds and compositions for controlling cell proliferation and treating cancer, regulating Aurora kinase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0366]
[0367] Synthesis of 2-chloro-4-(2-chloro-pyridin-3-yl)-[1,3,5]triazine
[0368] Step 1.2-Preparation of Chloro-Nicotinamidine
[0369] At 0°C, 2-chloro-3-cyanopyridine (5.0 g, 36 mmol) was dissolved in anhydrous EtOH (100 mL). HCl was bubbled into the mixture for 3 hours, the mixture was sealed, and centrifuged (about 8°C) overnight. After concentration, the residue was stirred with ammonium acetate (5.5 g) in 100 mL IpOH. After 12h, use concentrated NH 4 OH solution, adjust the pH to 9 (from 4), continue stirring for more than 2 days. The mixture was concentrated and subjected to flash chromatography (10:1:0.1CH 2 Cl 2 / MeOH / NH 4 OH) Purification. Trituration in hot tBuOMe / IpOH removed some residual amide by-products, and the product was obtained as a white solid.
[0370] Step 2: Amino-(2-chloro-pyridin-3-yl)-methylaminocyanide
[0371] Suspend 2-chloro-nicotinamidine (nicotinamidine) in 10 mL IpOH with 500 mg of solid cyanamide, and add 5% NaHCO to the stirred solid....
Embodiment 2
[0375]
[0376] Synthesis of [4-(2-Chloro-pyridin-3-yl)-[1,3,5]triazin-2-yl]-methyl-amino
[0377] Add methylamine to a solution of 2-chloro-4-(2-chloro-pyridin-3-yl)-[1,3,5]triazine (10.0g, 44.0mmol) in 55ml dichloromethane at 0°C (45ml, 88.0mmol) in 2.0MTHF. After stirring for 18 hours at room temperature, the mixture was diluted with acetone, filtered through a silica gel pad, and concentrated to obtain the desired product. MS m / z=222[M+H] + . C 9 H 8 ClN 5 Calculated value: 221.65.
Embodiment 3
[0379]
[0380] Synthesis of 4-(2-chloro-pyridin-3-yl)-pyrimidine
[0381] Step 1. Preparation of 5-(2-chloro-pyridin-3-yl)-3-dimethylamino-propenone
[0382] In a drying tube at 85°C, mix 1-(2-chloro-pyridin-3-yl)-ethanone (21.7g, 139mmol) in 46mL N,N-dimethylformamide dimethylacetal (42g, 350mmol) ) Is heated for 1.5h, and then concentrated. The residue was purified by suction filtration chromatography (using 150g silica gel in a Buchner funnel, quickly collect the fractions, use 10:1, then use 5:1 CH 2 Cl 2 / IpOH) to obtain a yellow solid product. MS m / z=211[M+H] + . C 10 H 11 ClN 2 O calculated value: 210.66.
[0383] Step 2. Preparation of 4-(2-chloro-pyridin-3-yl)-pyrimidine
[0384] Use 500mL IpOH bath at room temperature as the cold source, at room temperature, N 2 Next, small pieces of metallic sodium (8.3 g, 360 mmol in total) were added intermittently to 400 mL of anhydrous methanol, and sodium methoxide was generated over 1.5 hours. Formamidine acetate (42.7 g, 410 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com