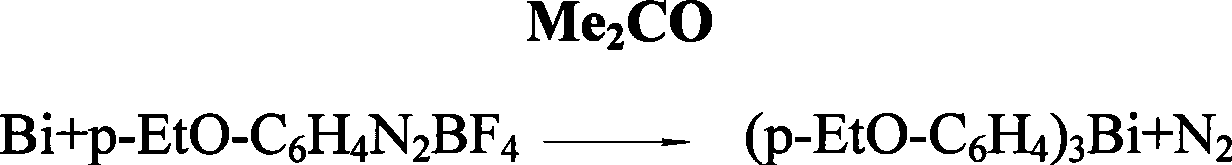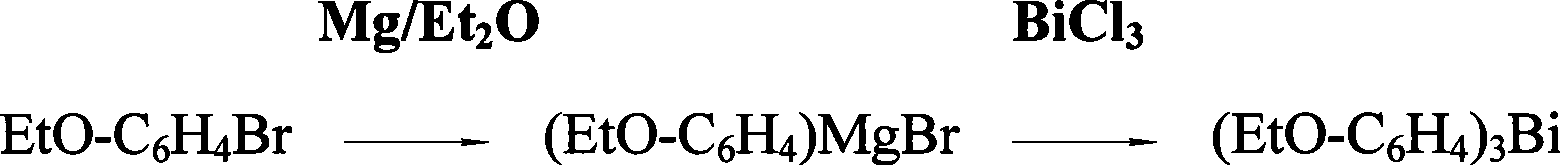Preparation of tri(alkoxyphenyl)bismuth compounds
A technology of alkoxyphenyl and alkoxybromobenzene, which is applied in the field of preparation of tri-bismuth series compounds to achieve the effects of reducing pollution, saving costs, and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The preparation of embodiment 1. three-(p-ethoxyphenyl) bismuth
[0020] In a 1L dry reaction flask, add 29.3 grams (1.205mole) of magnesium chips and 80ml of THF (cover the magnesium chips), add 242.3 grams (1.205 mole) of p-EtO-C in the high dropping funnel 6 h 4 A mixed solution of Br and 230ml THF was added dropwise with slow stirring under nitrogen protection, while heating carefully to initiate the reaction. After the reaction occurs, control the rate of addition to keep the reaction in a slightly boiling state. The reaction temperature can reach 90 ° C ~ 120 ° C. After about 1 hour, the dropwise addition is completed and the reaction is continued for about 2.5 hours at the temperature of an oil bath at 120 ° C to fully generate p-EtOC 6 h 4 MgBr Grignard reagent. Then, 123 grams (0.39 mole) of anhydrous BiCl in the high-position dropping funnel was mixed under stirring condition 3 The emulsified solution prepared with 240ml THF was added dropwise to the Grign...
Embodiment 2
[0021] Embodiment 2. the preparation of three-(ortho-ethoxyphenyl) bismuth
[0022] In a 1L dry reaction flask, add 13.5g (0.556mole) magnesium chips and 60ml THF (cover the magnesium chips), add 111.8g (0.556mole) o-EtO-C 6 h 4 A mixed solution of Br and 150ml THF was added dropwise under nitrogen protection with slow stirring and carefully heated to initiate the reaction. After the reaction occurs, control the dripping speed to keep the reaction in a slightly boiling state. The reaction temperature can reach 90 ° C ~ 120 ° C. It takes about 0.5 hours to complete the dropwise addition and continue to react for about 1.5 hours at 120 ° C oil bath temperature to fully generate o- EtOC 6 h 4 MgBr Grignard reagent. Then 57g (0.181mole) of anhydrous BiCl in the high position dropping funnel was mixed under stirring condition 3 The emulsified solution prepared with 150ml THF was added dropwise to the Grignard reagent for metal transfer reaction, and the addition was completed ...
Embodiment 3
[0023] Embodiment 3. the preparation of three-(m-ethoxyphenyl) bismuth
[0024] In a 1L dry reaction flask, add 25.2g (1.0356mole) magnesium chips and 80ml THF (cover the magnesium chips), add 208.1g (1.035mole) m-EtO-C 6 h 4 A mixed solution of Br and 200ml THF was added dropwise under nitrogen protection with slow stirring and carefully heated to initiate the reaction. After the reaction occurs, control the rate of addition to keep the reaction in a slightly boiling state. The reaction temperature can reach 90°C to 120°C. The dropwise addition is completed in about 1 hour, and the reaction is kept at 120°C for about 2.5 hours in an oil bath to fully generate m-EtO- C 6 h 4 MgBr Grignard reagent. Then 105.6g (0.335mole) of anhydrous BiCl in the high position dropping funnel was mixed under stirring condition 3 Add the emulsified solution prepared with 210ml THF dropwise into the Grignard reagent for the metal transfer reaction, finish the addition in about 1 hour and kee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com