Finasteride and doxazosin compound controlled release capsule and use thereof
A technology of doxazosin and finasteride, which is applied in the field of medicine, can solve problems such as unovercome adverse reactions of combined medication and increased toxicity of drugs, so as to reduce the time of taking medicine, reduce toxic and side effects, and improve compliance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
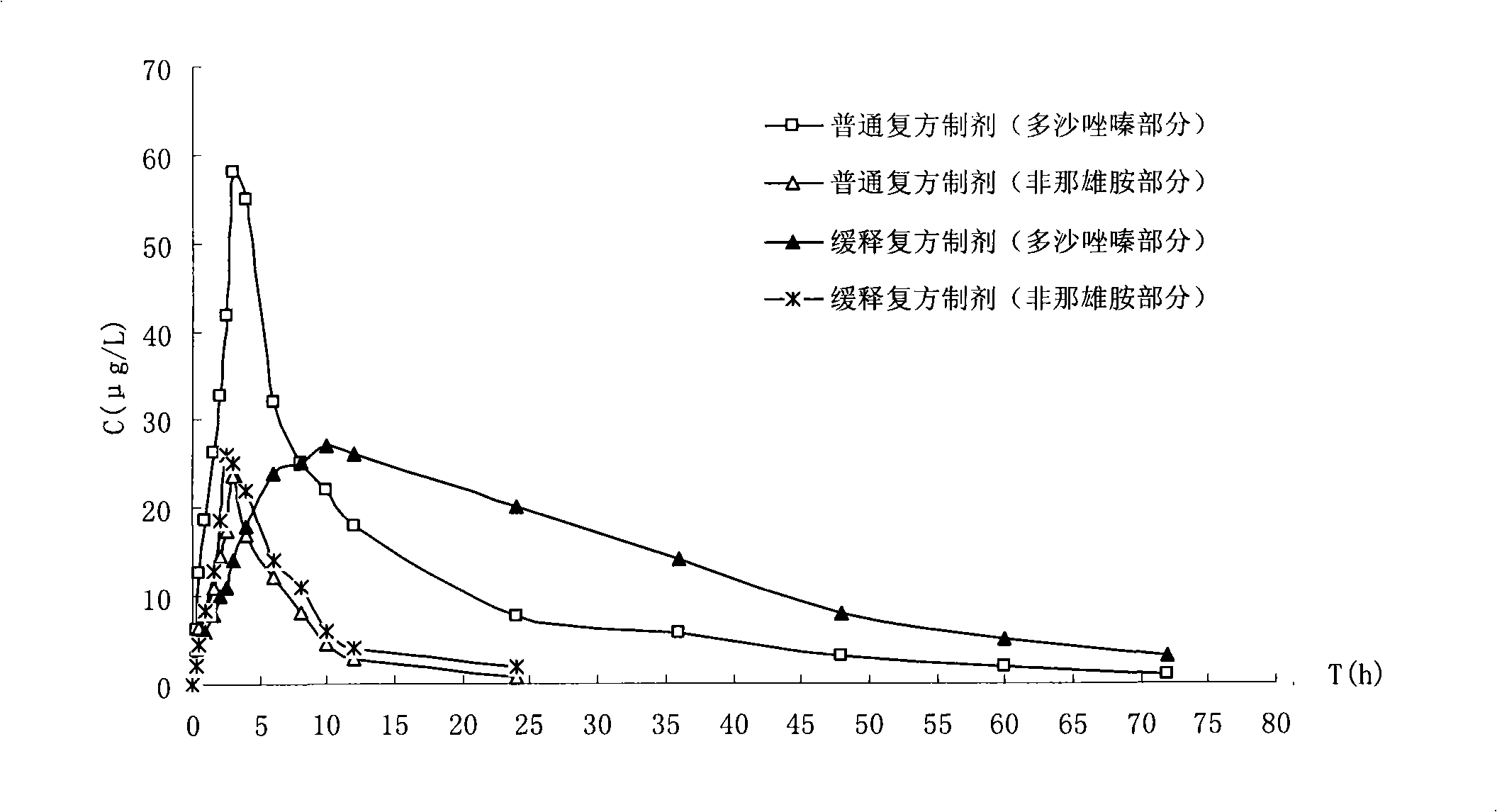
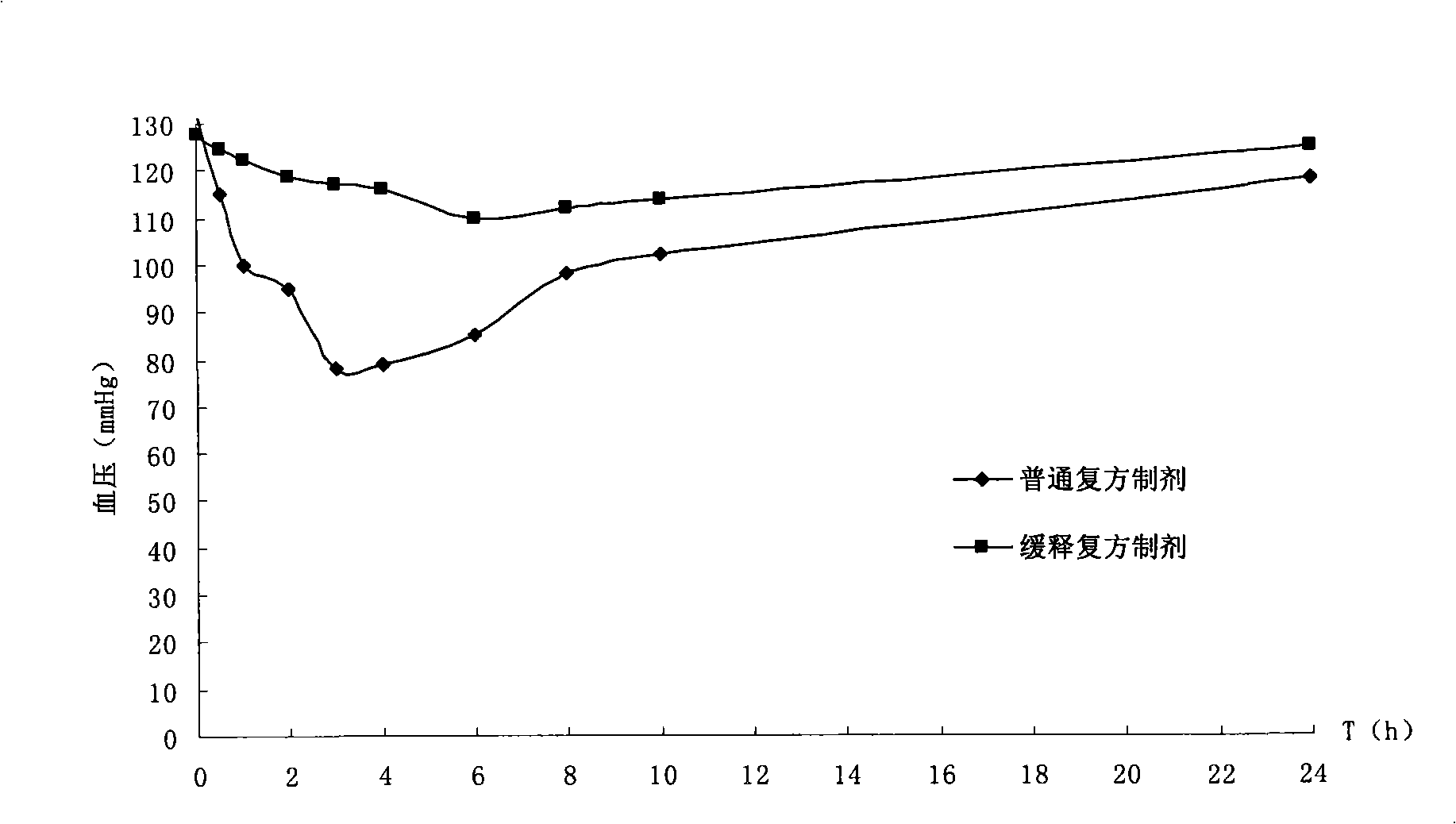
Image
Examples
Embodiment 1
[0025] Embodiment 1: Preparation of finasteride immediate-release pellets
[0026] Finasteride micropellet coating preparation process: mix the drug, binder, co-solvent and organic solvent in the formula (according to the formula) to make a coating solution; the above-mentioned coating solution will be used to coat the blank in a fluidized bed Sugar pellet core coating. During coating, the drug content of finasteride in the pellets can be controlled at 1-60 wt%.
[0027] Here, the drug is finasteride; the binder can be selected from starch slurry, methylcellulose, hydroxypropylcellulose, hypromellose, ethylcellulose, povidone, gelatin, polyethylene glycol, etc. One or more of them are mixed; when the degree of drug solubility is not enough, a cosolvent can be added, and the cosolvent is selected from sodium lauryl sulfate, Tween, Span, phospholipids, polyoxyethylene poly One or more mixtures of oxypropylene copolymer, glyceryl stearate, sucrose fatty acid ester, etc.; the or...
Embodiment 2
[0052] Embodiment 2, the preparation of doxazosin sustained-release pellets
[0053] Coating preparation process of doxazosin sustained-release pellets: firstly mix the drug with fillers and binders, then perform wet granulation, then extrude and spheronize to prepare drug pellets. After the drug pellets are dried, use a fluidized bed to The coating solution is used for coating, and the weight gain of the coating is 5-17%. The doxazosin drug contained in the pellets can be controlled in an amount of 1-60 wt%.
[0054] Here, the drug in the drug pellets is doxazosin or its pharmaceutically acceptable salt, and the pharmaceutically acceptable salt can be hydrochloride, sulfate, mesylate, maleate, preferably doxazosin mesylate; filling The agent is selected from one or more of lactose, mannitol, xylitol, sorbitol, powdered sugar, dextrin, starch, pregelatinized starch, microcrystalline cellulose, inorganic salts, etc., and the binder is selected from One or more of starch slurr...
Embodiment 3
[0123] Example 3: Preparation of Finasteride Doxazosin Controlled Release Capsules
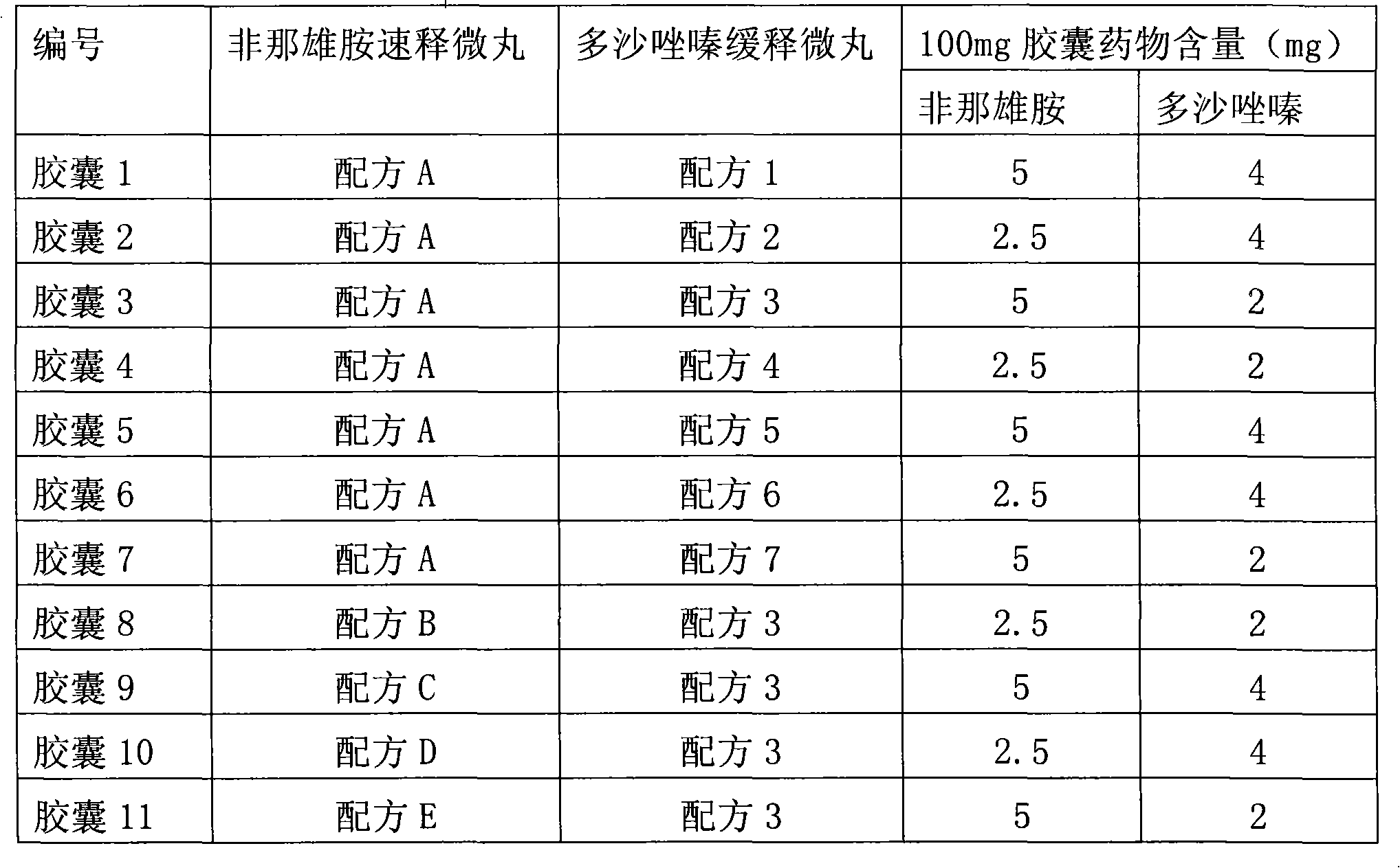
[0124] The two kinds of micropills prepared in Examples 1 and 2 were respectively mixed uniformly according to the amount of the two kinds of medicines according to the combination method in Table 1, and then packed into empty capsules, and the loading amount of each capsule was 100 mg.
[0125] Table 1: Finasteride-doxazosin compound controlled-release capsules
[0126]
[0127] Experimental part
[0128] Experiment 1. The effect of finasteride doxazosin with different ratios on benign prostatic hyperplasia
[0129] 80 adult SD male rats, weighing 70-110g. They were randomly divided into 8 groups according to Table 2. Except the normal group, the rats in the other 7 groups were castrated respectively: the rats were anesthetized by intraperitoneal injection of 3.0% chloral hydrate (10ml / kg), underwent aseptic surgery through the scrotum, and removed both testes. Start medication 7 day...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com