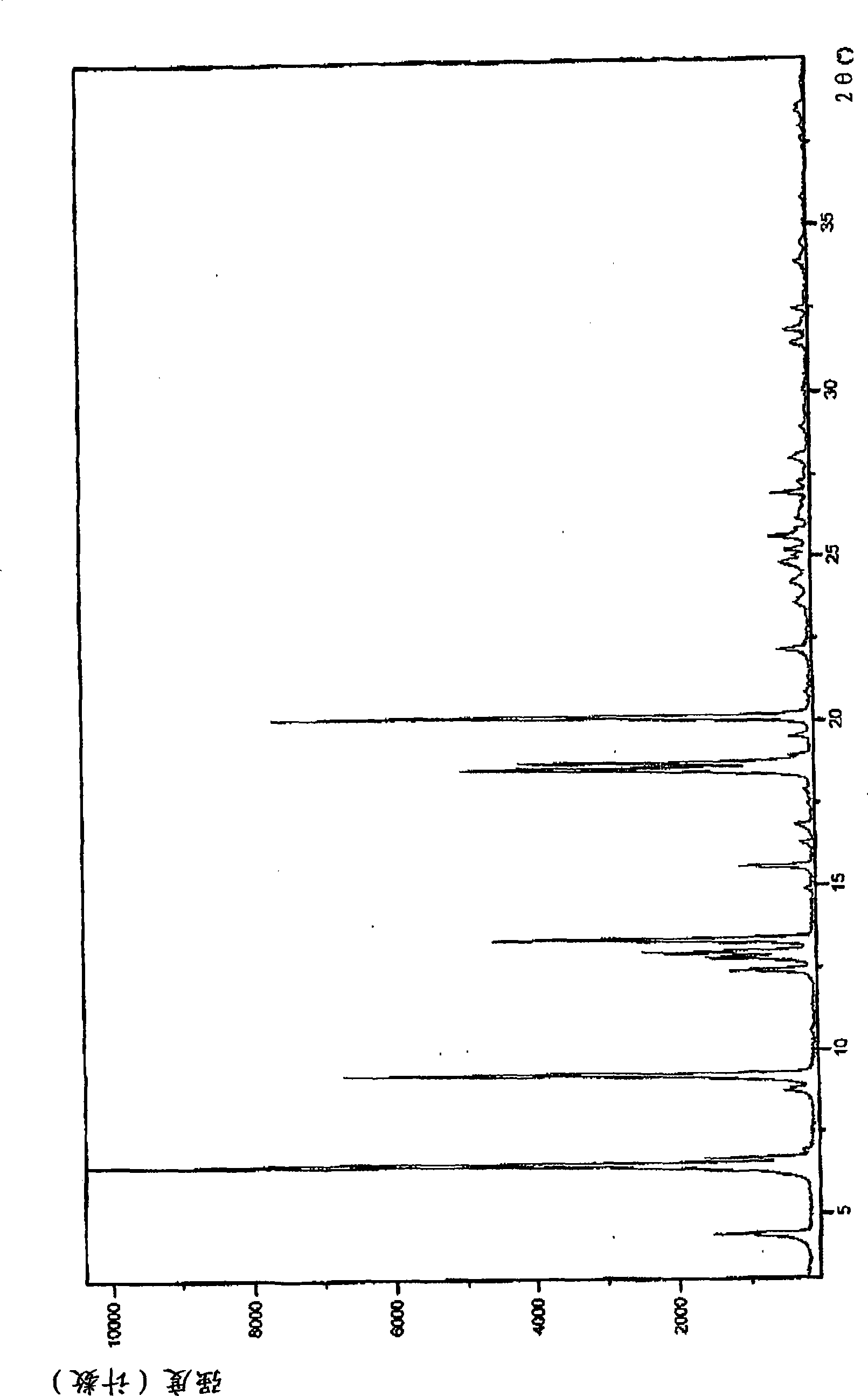
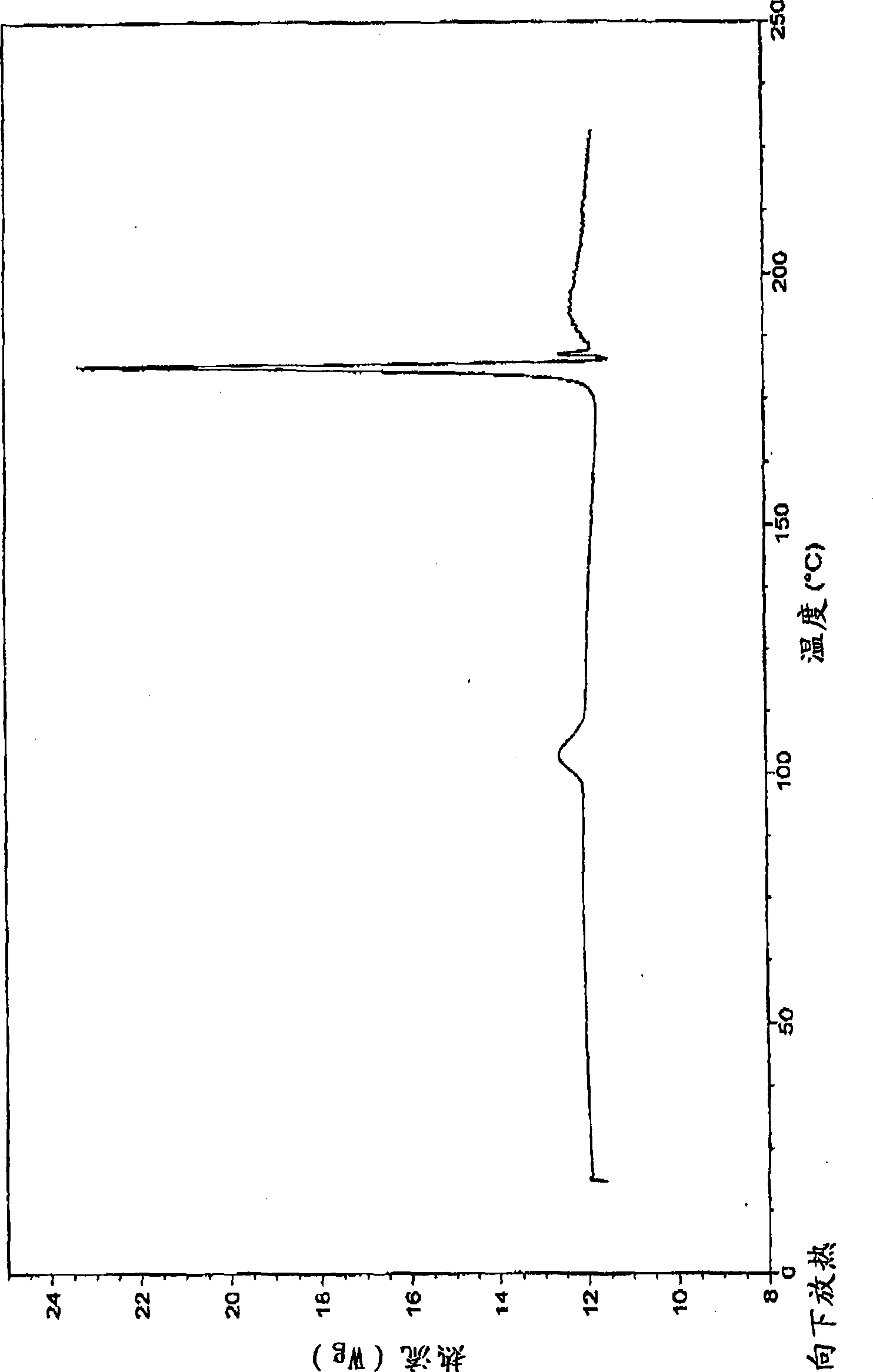
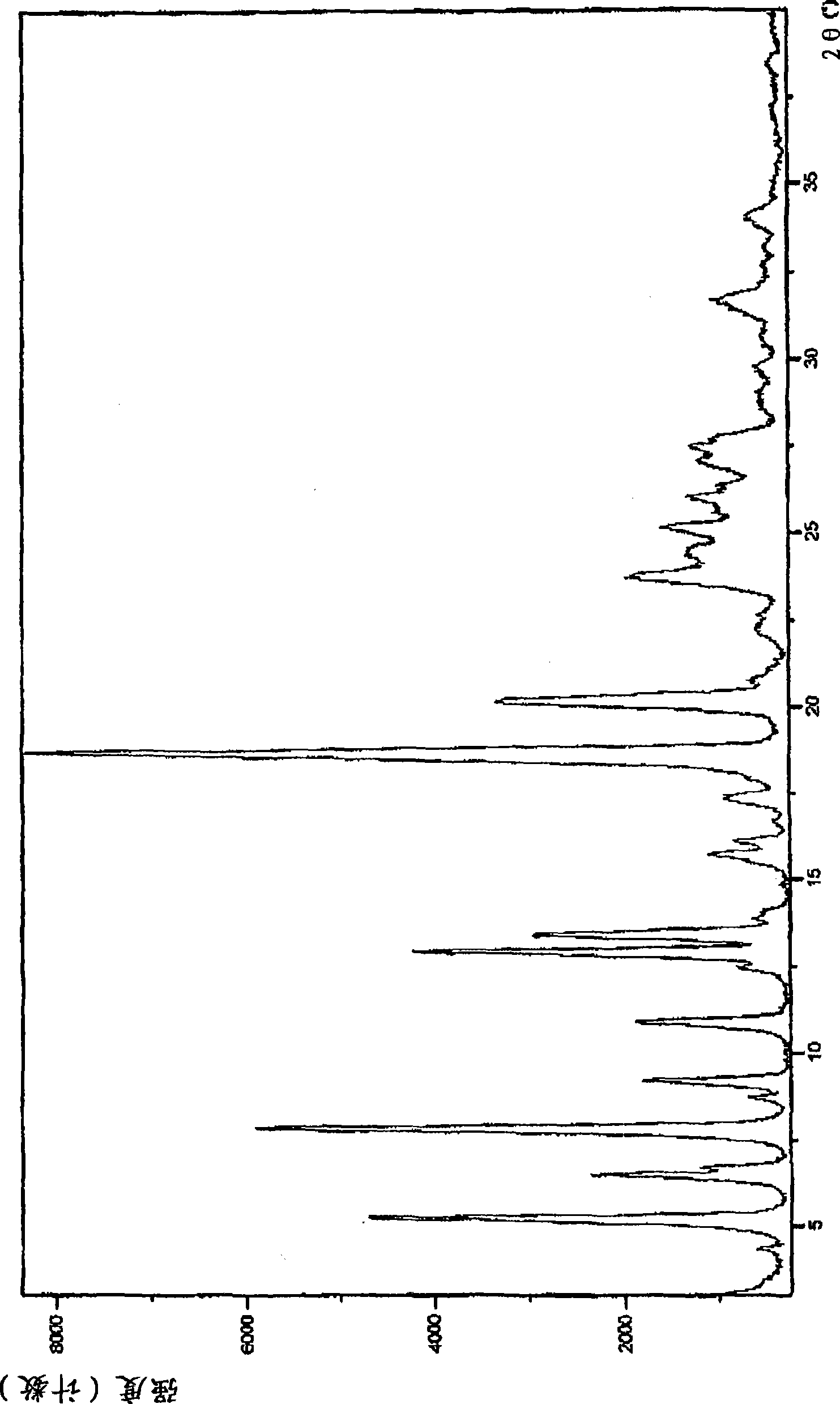
Pharmaceutically acceptable salt and polymorphic forms of Flupirtine maleate
A technology of flupirtine maleate and polymorphs, applied in the field of polymorphs of flupirtine maleate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0046] As used herein, the term "therapeutically effective amount" refers to the amount of the polymorphic form of flupirtine maleate of the present invention that is capable of preventing, ameliorating or eliminating the disease state that is centrally responsible for its administration. Disease state with opioid analgesics, muscle relaxants, functional NMDA antagonists, or substances that increase expression of the protein Bcl-2.
[0047] "Pharmaceutically acceptable" means that the carrier, diluent or excipient is compatible with the polymorphic form of flupirtine maleate of the present invention and is harmless to the recipient thereof.
[0048] In the pharmaceutical composition of the present invention for oral, sublingual, subcutaneous, intramuscular, intravenous, topical, intratracheal, intranasal, transdermal or rectal administration, the polymorphic flupirtine maleate of the present invention The unit dosage forms mixed with conventional pharmaceutical carriers are ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com