Dihydropyridine compounds and compositions for headaches
A kind of technology of dihydropyridine and compound, applied in the field of dihydropyridine compound and composition for headache
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
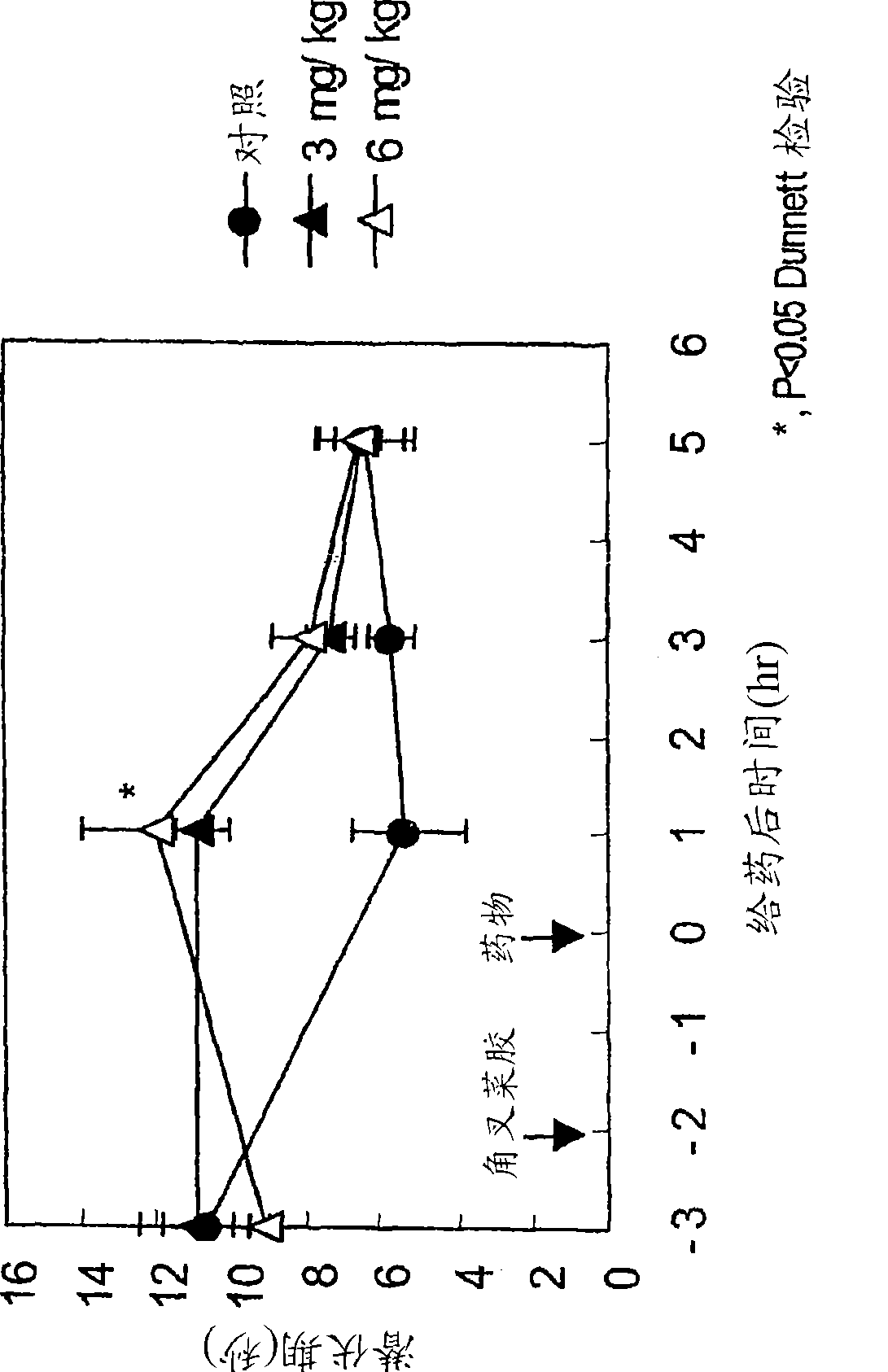
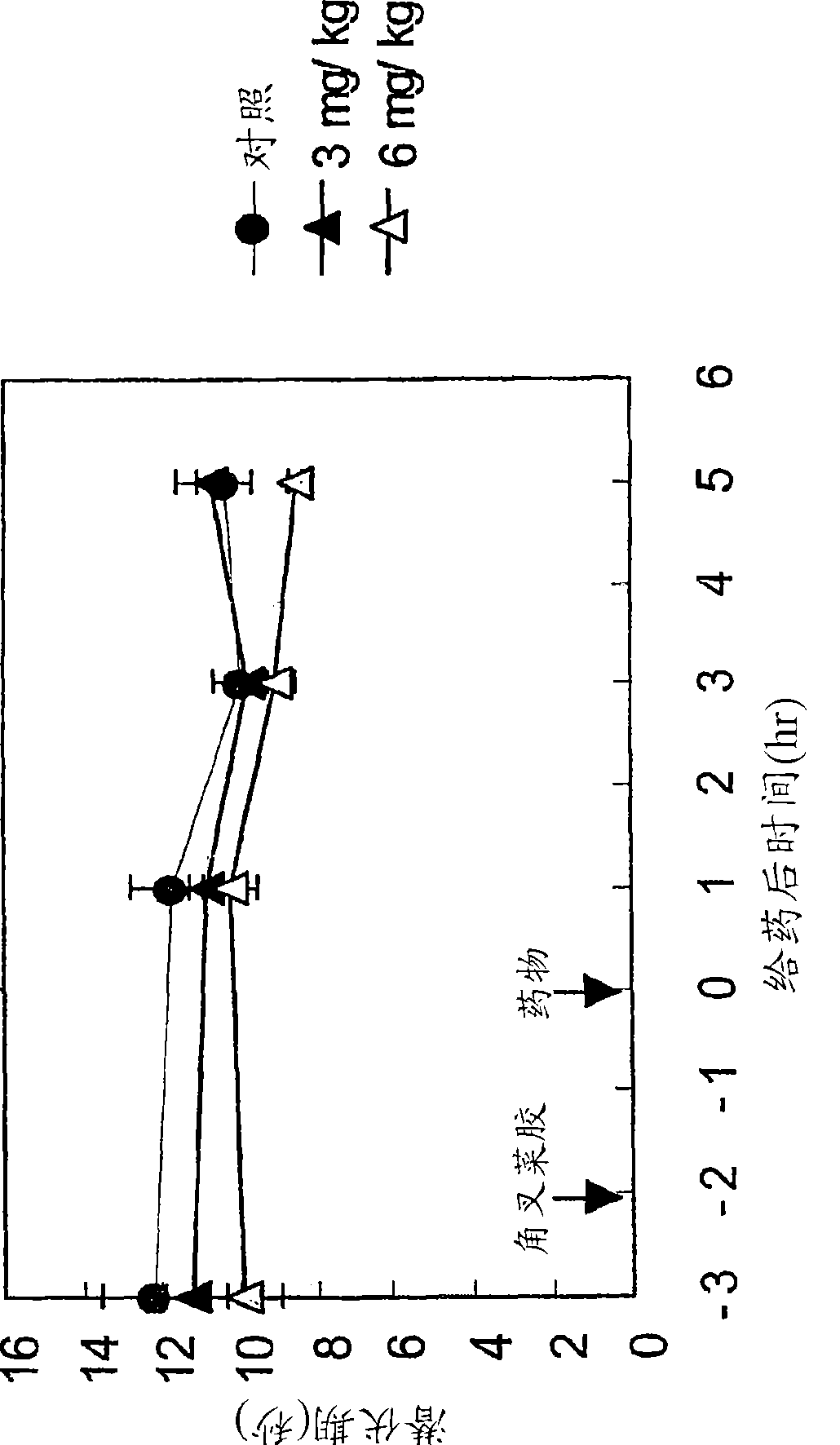
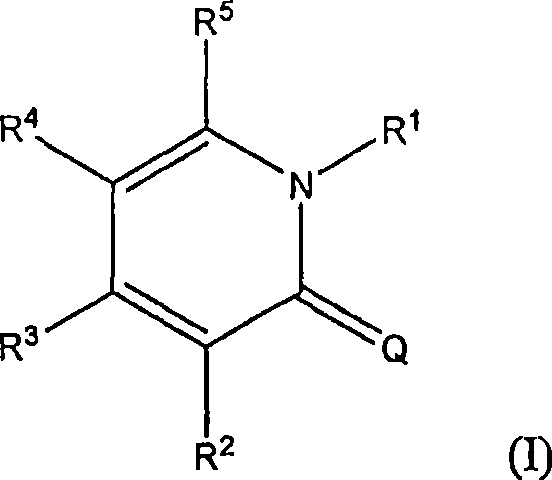
[0181] Anti-migraine agents are commonly evaluated using the carrageenan-induced thermal hyperalgesia model (Bingham et al., Experimental Neurology, 167:65-73 (2001); Daher et al., Life Sciences, 76:2349-2359 (2005)) . Antimigraine properties of compound A (i.e., 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one) in response to thermal hyperalgesia Evaluated in the following rat carrageenan-induced inflammatory pain model.
[0182] Male Wistar rats (5 / group) were used in the experiments. The withdrawal latencies of both hind paws of rats to avoid thermal noxious stimuli were measured using TAIL FLICK 7360 (Ugo Basile, Italy). 1% carrageenan was injected into the sole of the right hind paw of the rat. 2 hours after carrageenan injection, rats were orally administered 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridine-2 - ketone or vehicle. The withdrawal latencies of the two hind paws of the heat-stimulated rats were measured at 1, 3 and 5 hours a...
Embodiment 2
[0186] To evaluate the efficacy and For safety, a randomized double-blind placebo-controlled, multicenter parallel group study was conducted.
[0187] The primary efficacy endpoint was to evaluate the effect of 23-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one hydrate in reducing migraine , based on the compound's change in frequency of migraine sessions per 28 days during treatment relative to baseline. A migraine period was defined as the onset, end, or recurrence of migraine within 24 hours. If the migraine lasted longer than 24 hours, it was considered a new migraine period.
[0188] The second objective of this study was to evaluate the role of 23-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one hydrate in migraine Safety and tolerability in patients; characterizing 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one hydrate in Pharmacokinetics in a patient population; determination of plasma concentrations of 3-(2-cyanoph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com