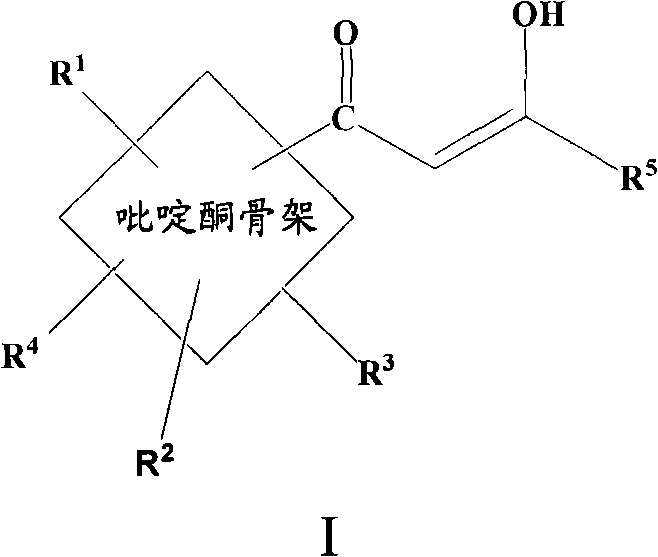
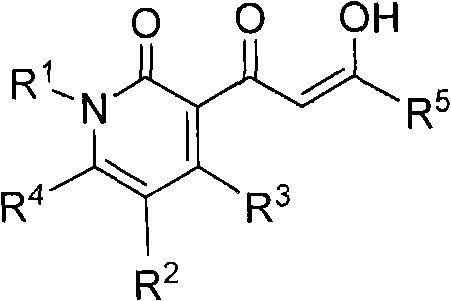
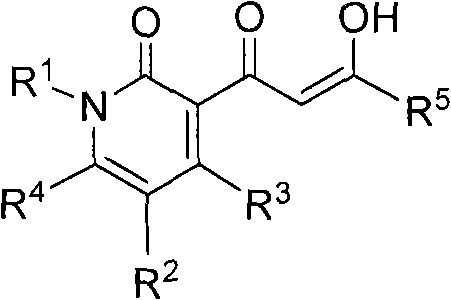
Pyridinone diketo acids: inhibitors of HIV replication in combination therapy
A technology of composition and compound, which is applied in the field of treatment of human HIV infection and combination therapy, and can solve problems such as no described compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0938] 4-(1,5-Dibenzyl-1,2-dihydro-2-oxopyridin-3-yl)-2-hydroxy-4-oxobut-2enoic acid (8).
[0939] The related process (1) is as follows.
[0940]
[0941] Process 1
[0942]Step 1: 5-Benzylpyridin-2-amine (2).
[0943]
[0944] A mixture of pyridin-2-amine 1 (14.1 g, 149.8 mmol) and benzyl chloride (36.0 g, 284.6 mmol) was heated to 180° C. until the mixture boiled [Kowalski, J. Heterocycl. Chem. 28, 875-879 (1991 )]. The temperature was then ramped up to 250°C over 3 hours and held there for 24 hours. After cooling, the reaction mixture was washed out of the flask with MeOH (60 mL) and washed with 10% NH 4 Aqueous OH solution (40 mL) was used for treatment. After adding water (200mL), with CHCl 3 (2×200mL) extracted the resulting oil with anhydrous Na 2 SO 4 dried, and distilled off CHCl 3 . The residue was separated by distillation under reduced pressure. Fractions collected at 130-135 °C / 1 mm Hg were further purified by flash chromatography on silica gel...
Embodiment 2
[0964] 4-(1,5-Dibenzyl-1,4-dihydro-4-oxopyridin-3-yl)-2-hydroxy-4-oxobut-2enoic acid (16).
[0965] The related process (2) is shown below.
[0966]
[0967] Process 2
[0968] Step 1: 3,5-Dibromo-pyridin-4-one (10)
[0969]
[0970] To an ice-cold solution of pyridin-4-one 9 (6.98 g, 73.4 mmol) and KOH (9.52 g, 146.8 mmol) in water (140 mL) was added bromine (7.58 mL, 147.5 mmol) dropwise over 30 minutes [Spivey, et al. , J. Org. Chem. 65, 3154-3159 (2000)]. 30 minutes after the addition, the precipitate was filtered, washed with copious amounts of water, and dried in vacuo. Yield 16.17 g (87%), yellow solid, mp 320°C (sublimation). 1 H NMR (DMSO-d 6 , 500MHz): δ12.3(s, 1H), 8.26(s, 2H). 13 C NMR (DMSO-d 6 , 125MHz): δ167.5, 138.2, 138.2, 111.8, 111.8.
[0971] Step 2: 3-Bromo-5-(hydroxy-phenyl-methyl)-pyridin-4-one (11)
[0972]
[0973] To a heterogeneous mixture of 3,5-dibromo-pyridin-4-one 10 (0.313 g, 1.24 mmol) in anhydrous THF (4 mL) was added phenylm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com