Betulinol derivatives as anti-HIV agents
a technology of betulinol and derivatives, which is applied in the direction of biocide, immunological disorders, drug compositions, etc., can solve the problems of limited anti-hiv-1 activity, difficult manufacturing, and high cost of drugs, and achieve superior anti-hiv-1 activity and anti-hiv-1 activity. superior
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation and Structure of Betulinol and its Derivatives
[0417] Betulinol is isolated from the non-saponofiable fraction of the crude sulfate soap prepared by boiling the outer bark of the white birch tree in NaOH, Na2SO4, Na2SO3, and Na2S2O3 at 110-120° C. Betulinol is then crystallized by using solvents such as acetone, ethyl acetate, isopropanol, butanol, ethanol, etc. The chemical structure of betulinol is:
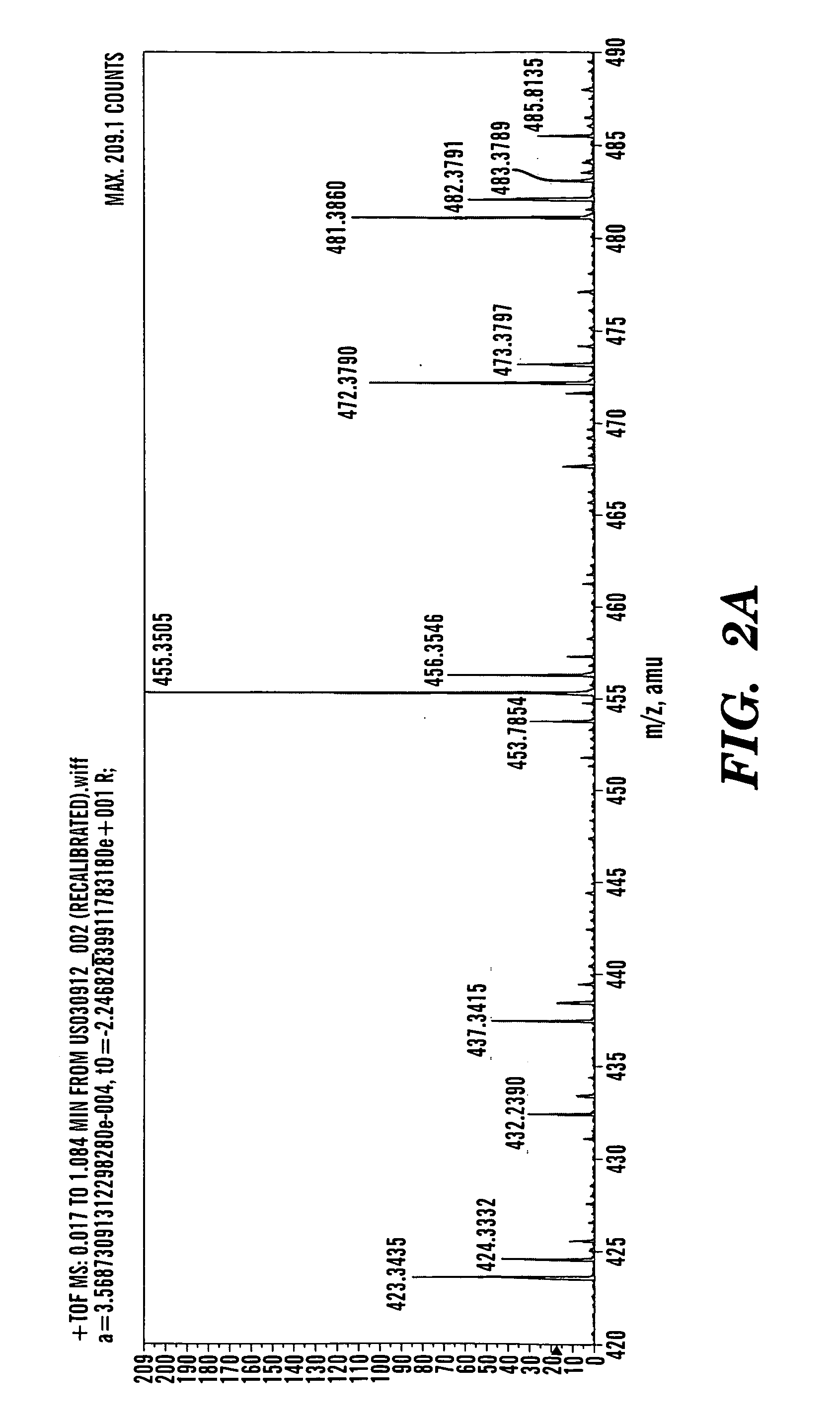
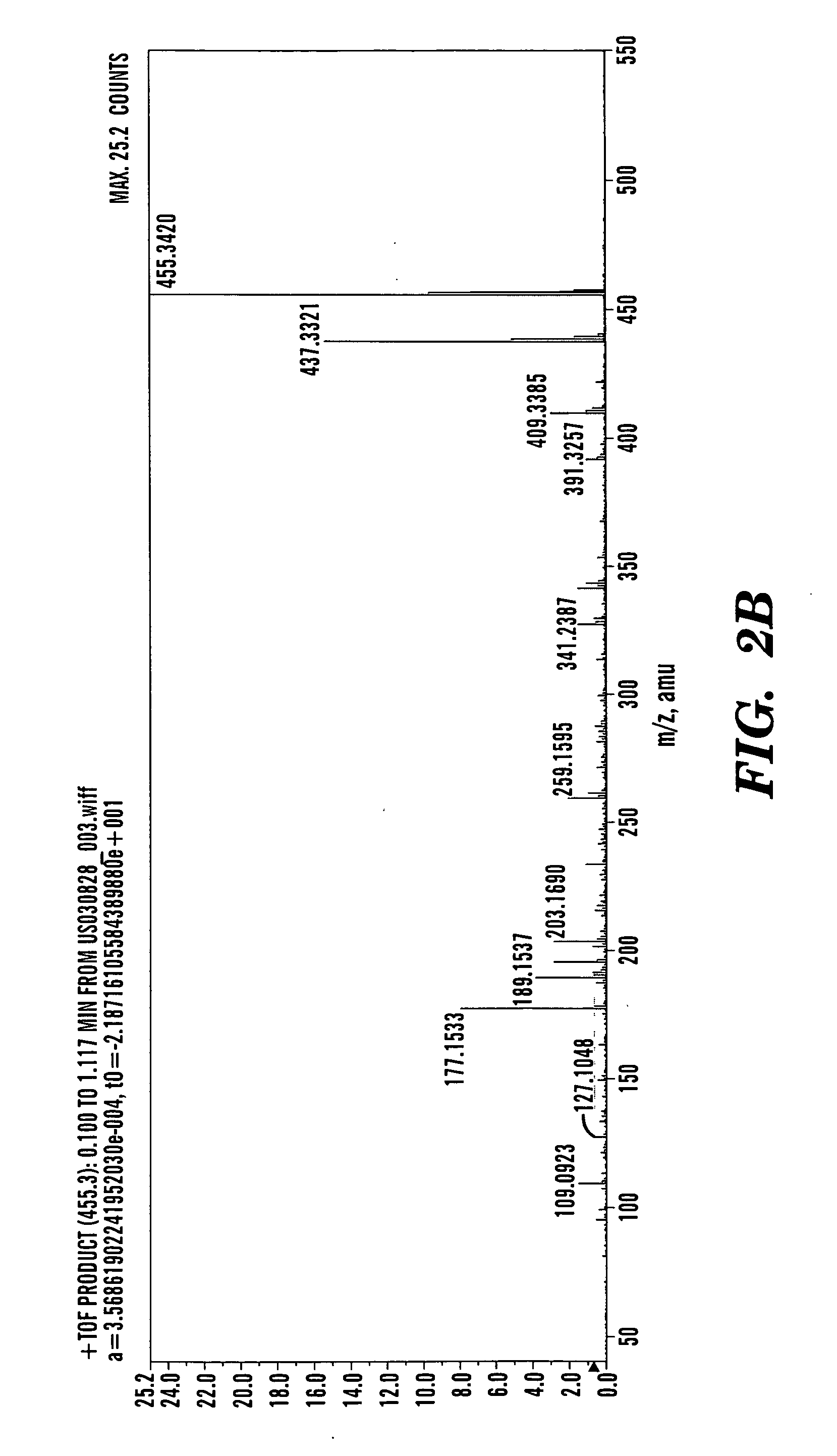
[0418] Betulinol is a non-steroidal, lupeol-derived, pentacyclical, lupan-row alcohol of the group of styrenes. Betulinol (also known as betulin) has a chemical formula of C30H50O2 and a molecular weight of 442.7 g / mol. The structure of betulinol is based on a 30-carbon skeleton of four, six-member rings and one, five-member E-ring containing an a-isopropyl group. The structural component of betulinol has a primary and a secondary hydroxyl group at C-3 and C-28. Betulinol has three sites (C-3, C-20, and C-28) where chemical modification can occur to yield derivatives. With t...
example 2
Synthesis of Betulonic Acid from Betulinol
[0420]
[0421] In a typical procedure, 500 mg of betulinol (1) was added to a suspension of 1.2 g of freshly activated 4 Å molecular sieves, 1.2 g of celite, 1.2 g of florisil, 500 mg of sodium acetate, and 1.2 g of pyridinium chlorochromate in 25 mL of CH2Cl2. The mixture was stirred for 2 hrs, and then filtered through a 2.5×15 cm column silica gel of 230-400 mesh and 60 Å (HF-254, E. Merck). The filtrate was evaporated in vacuum. The residue was subjected to column chromatography of silica gel to recover 370 mg of betulone aldehyde (2) as white solid. This betulone aldehyde was dissolved in 17 mL CH3CN—H2O containing 877 mg of NaH2PO4.H2O and cooled to 0-5° C. 220 μL of 30% of aqueous H2O2 and 200 mg of NaClO2 dissolved in 16 mL water were added in tandem. The mixture was brought up to room temperature and stirred for one hour. The reaction was quenched by the addition of 380 mg of Na2S2O5. The betulonic acid was extracted with 300 mL ethy...
example 3
Results of Chemistry Characterizations: GC
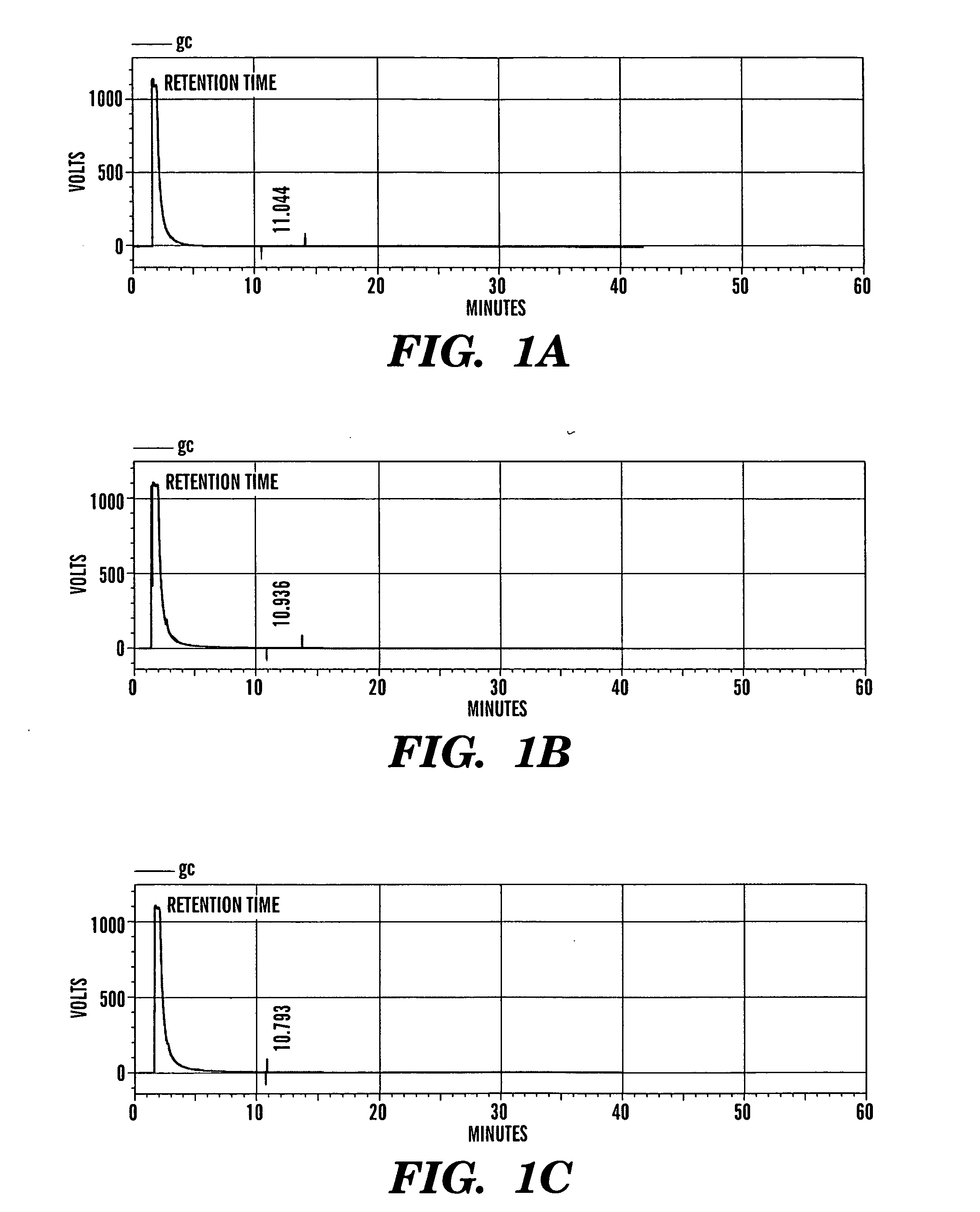
[0422] The purity of betulonic acid and its derivatives was studied by taking gas chromatographic profiles. 8 μL of each sample was injected to yield the following retention time (tR):
TABLE 2Retention Times from Gas Chromatogram ProfilesSampleRetention Time (tR)Betulonic Acid11.044Monomer10.936Dimer10.793
[0423] FIGS. 1A-C show a typical chromatogram of betulonic acid and its derivatives with their corresponding retention time. Close examination of these chromatograms reveals that the conjugated betulonic acid monomer and dimer, gave neat chromatograms.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com