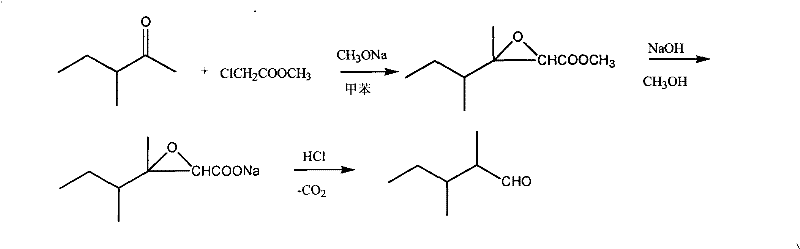
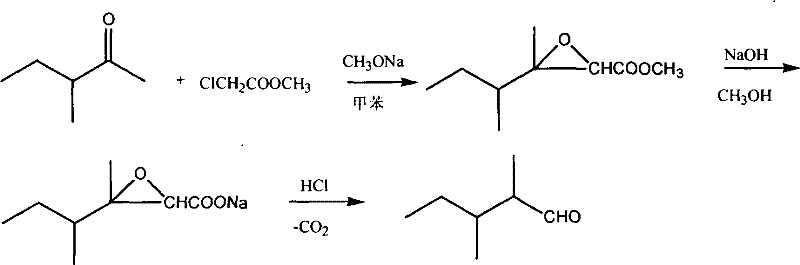
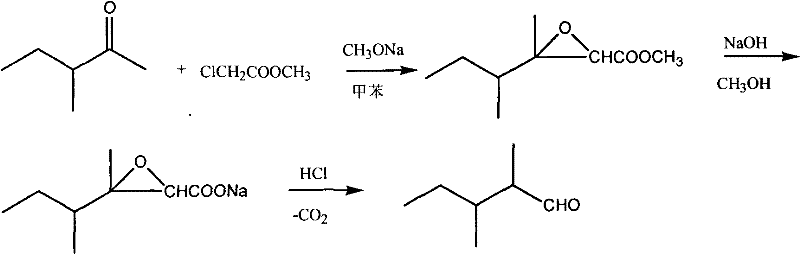
Synthetic method of 2,3-dimethylpentanal
A technology of dimethylvaleraldehyde and synthetic methods, applied in the preparation of heterocyclic compounds, organic chemistry, etc., can solve the problem of high equipment requirements, and achieve the effects of simple equipment, low production cost, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 1-liter flask equipped with a mechanical stirrer, a thermometer, and a solid addition funnel, add 90 g (0.9 mol) of 3-methyl-2-pentanone, 108.5 g (1 mol) of methyl chloroacetate and 100 mL of toluene, and cool the reaction solution to -20°C, add 64.8g (1.2mol) of sodium methoxide, control the reaction temperature to about -15°C, and complete the addition in about 3 hours.
[0029] After the addition, continue to keep warm and stir to react for 2 hours, then stir at room temperature for 1 hour, add dropwise 72g of sodium hydroxide and 400mL of methanol solution, and react at room temperature for 4 hours.
[0030] Recover methanol under reduced pressure at 20KPa. When no more distillate comes out, add 300mL of ice water, stir for about 5min, let stand for 30min, separate the toluene layer, acidify the water layer with concentrated hydrochloric acid to pH=1~2, and use Extract with ether three times, add 0.9g of copper powder, recover the solvent, and collect fractions...
Embodiment 2
[0032] In a 1-liter flask equipped with a mechanical stirrer, a thermometer, and a solid addition funnel, add 100 g (1 mol) of 3-methyl-2-pentanone and 108.5 g (1 mol) of methyl chloroacetate, and cool the reaction solution to -10°C. Add 64.8 g (1.2 mol) of sodium methoxide, control the reaction temperature to about -7°C, and complete the addition in about 3 hours.
[0033] After the addition, continue to keep warm and stir to react for 2 hours, then stir at room temperature for 1 hour, add dropwise 80 g of sodium hydroxide and 400 mL of methanol solution, and react at room temperature for 4 hours.
[0034] Recover methanol under reduced pressure at 20KPa. When no more distillate comes out, add 300mL of ice water, stir for about 5min, let stand for 30min, separate the toluene layer, acidify the water layer with concentrated hydrochloric acid to pH=1~2, and use Extract with ether three times, add 1g of copper powder, recover the solvent, and collect fractions at 72-75°C by 5KPa...
Embodiment 3
[0036] In a 1-liter flask equipped with a mechanical stirrer, a thermometer, and a solid addition funnel, add 90 g (0.9 mol) of 3-methyl-2-pentanone, 167 g (1 mol) of ethyl bromoacetate and 100 mL of toluene, and cool the reaction solution to - 0°C, add 95g (1.1mol) of sodium ethylate, control the reaction temperature at about 5°C, and complete the addition in about 3 hours.
[0037] After the addition, continue to insulate and stir the reaction for 2 hours, add dropwise 72g of sodium hydroxide and 400mL of methanol solution, and react at room temperature for 4 hours.
[0038] Recover methanol under reduced pressure at 20KPa. When no more distillate comes out, add 300mL of ice water, stir for about 5min, let stand for 30min, separate the toluene layer, and acidify the water layer with 25% sulfuric acid to pH = 1-2. Extract with ether three times, add 0.9g of copper powder, recover the solvent, and collect fractions at 72-75°C by 5KPa vacuum distillation to obtain 40g of 2,3-di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com