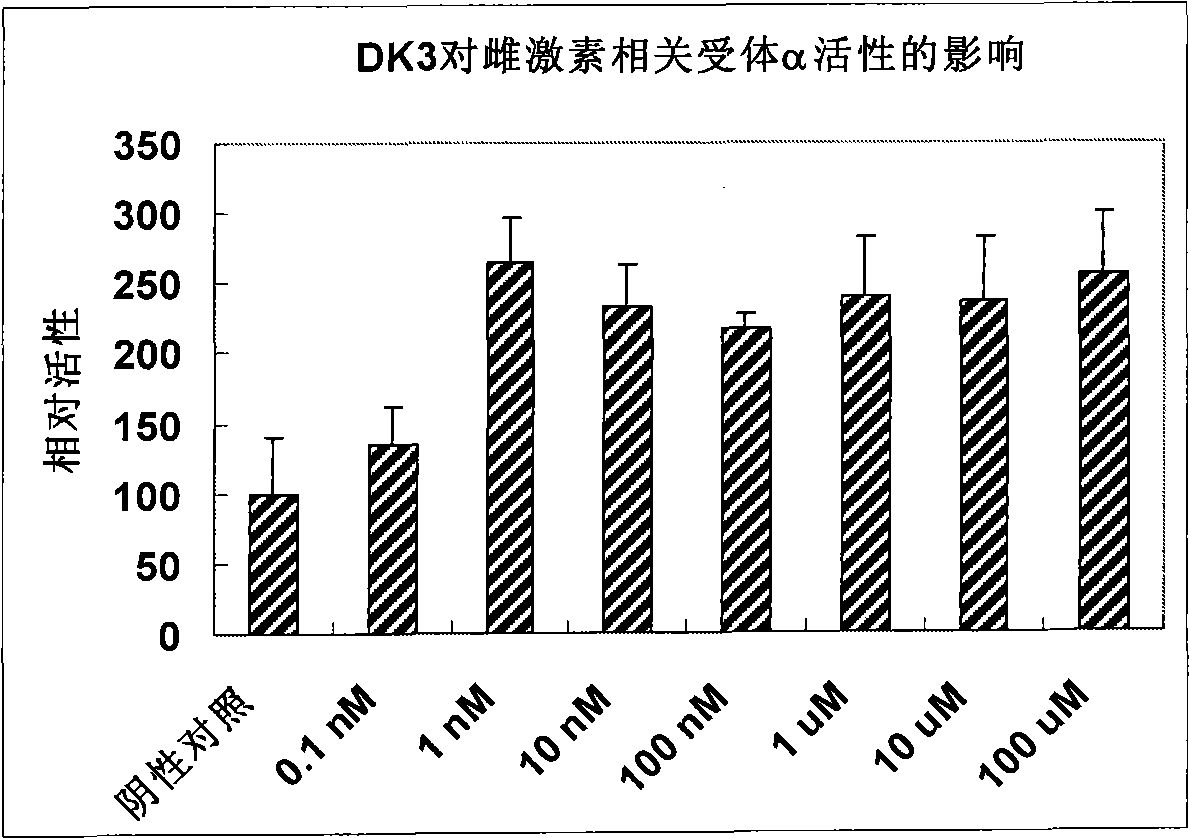
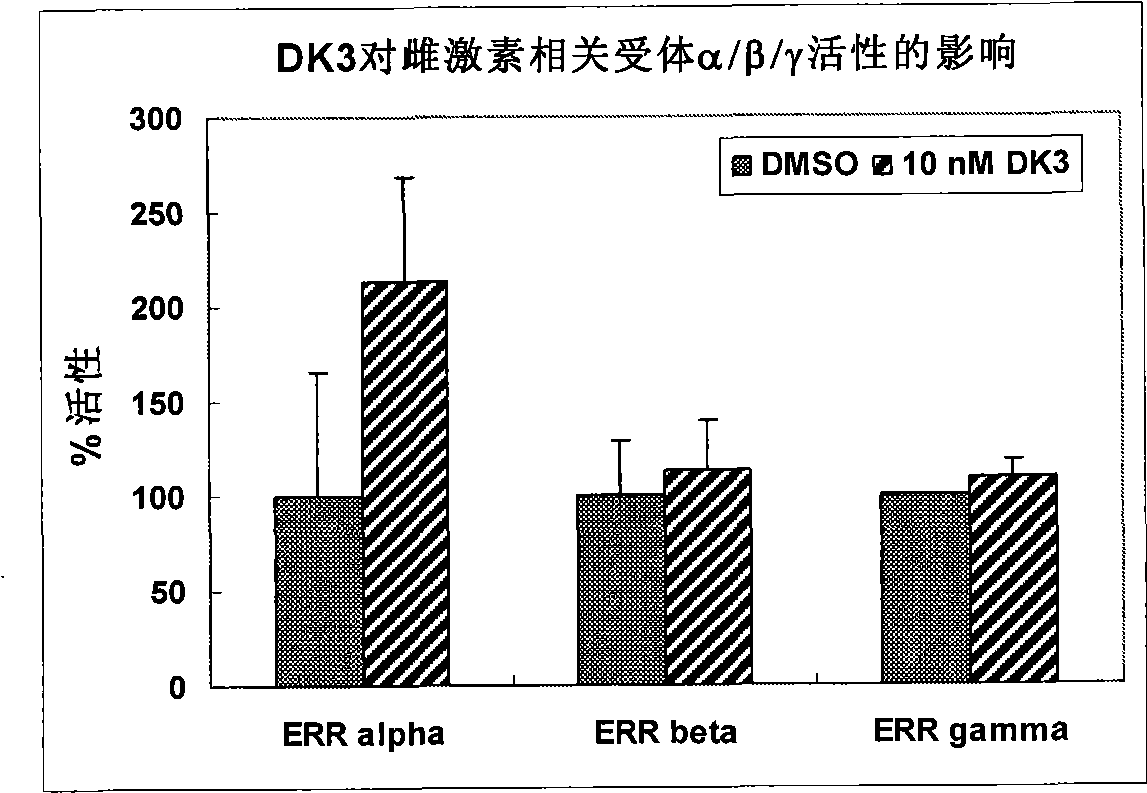
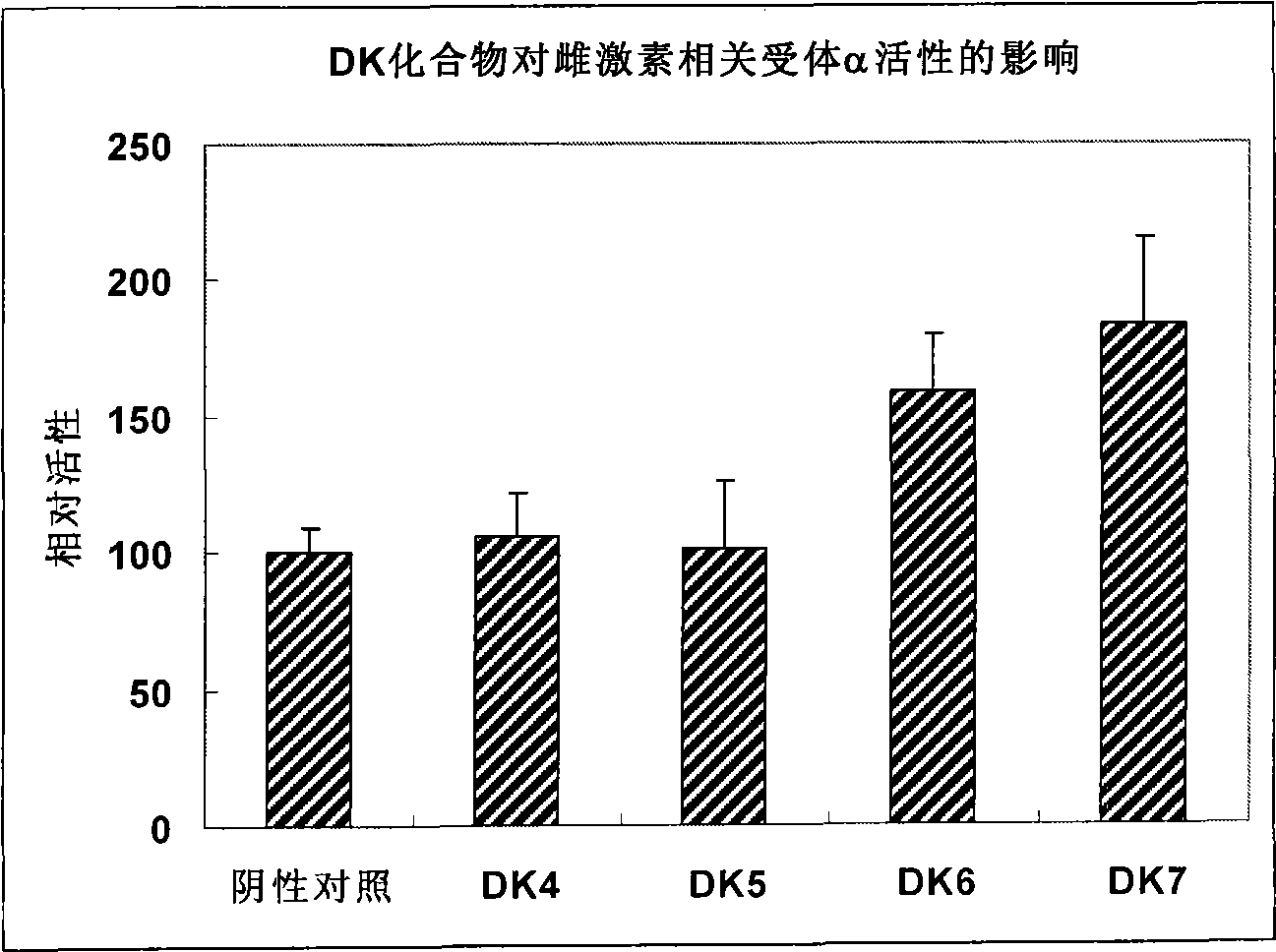
Compound used as a regulator of estrogen-related receptor and applications thereof
A compound, halogen technology, applied in digestive system, organic chemistry, drug combination, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0276] 7-methoxy-3-methyl-2-phenylquinazolin-4(3H)-one
[0277] (7-methoxy-3-methyl-2-phenylquinazolin-4(3H)-one)
[0278]
[0279] Step 1. 7-Methoxy 2-phenyl-4H-benzo[d][1,3]oxazoline-4-one (7-methoxy-2-phenyl-4H-benzo[d][1,3] oxazin-4-one)
[0280] Dissolve 2-amino-4-methoxybenzoic acid (2-amino-4-methoxybenzoic acid, 1.67 g, 10 mmol) in 10 mL of pyridine at room temperature, slowly add benzoyl chloride (1.7 g, 6 mmol, dissolved in 5 mL pyridine). After stirring at room temperature for 6 hours, the reaction mixture was poured into 50 g of ice water. Extracted with ethyl acetate and purified by column chromatography (petroleum ether: ethyl acetate = 5:1) to obtain 1.96 g of product (white solid, 77.5%).
[0281] Step 2. 7-methoxy-3-methyl-2-phenylquinazolin-4(3H)-one (7-methoxy-3-methyl-2-phenylquinazolin-4(3H)-one)
[0282] Mix 0.253 g (1.0 mmol) of the compound obtained above with 0.675 g methylamine hydrochloride (10 mmol) in 10 mL DMF, and heat to reflux for 5 hou...
Embodiment 2
[0286] 7-methoxy-3-methyl-2-(4-chlorophenyl)quinazolin-4(3H)-one
[0287] (2-(4-chlorophenyl)-7-methoxy-3-methylquinazolin-4(3H)-one)
[0288] The synthesis method is as in Example 1.
[0289] 1 HNMR (400MHz, CDCl 3 ), δ 8.21(d, J=8.4Hz, 1H), 7.53~7.10(m, 4H); 7.11~7.06(m, 2H), 3.91(s, 3H), 3.48(s, 3H);
[0290] MS (ESI), m / z: 300 (M + ).
Embodiment 3
[0292] 7-methoxy-3-methyl-2-(4-fluorophenyl)quinazolin-4(3H)-one
[0293] (2-(4-fluorophenyl)-7-methoxy-3-methylquinazolin-4(3H)-one)
[0294] The synthesis method is as in Example 1.
[0295] 1HNMR (400MHz, CDCl 3 ), δ 8.19(d, J=8.8Hz, 1H), 7.59~7.56(m, 2H); 7.26~7.19(m, 2H), 710(d, J=2.0Hz, 1H), 7.06(dd, J =2.0, 8.8Hz, 1H), 3.89(s, 3H), 3.46(s, 3H);
[0296] MS(ESI), m / z: 285(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com