Application for taking NO donor-type compounds as P-glycoprotein inhibitors and tumor multi-drug resistance reversal agents
A multi-drug resistance and multi-drug resistance technology, which is applied in the direction of antineoplastic drugs, pharmaceutical formulations, drug combinations, etc., to achieve the effects of reducing survival rate, increasing cell uptake, and obvious synergy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] NO-donating alkoxybiphenylcarboxylic acid compounds reduce the efflux ratio of the P-gp substrate digoxin. Taking the two compounds 14c and 14j as examples, the specific structures are as follows:
[0024]
[0025] experimental method:
[0026] Caco-2 cells by 1×10 5 Cells / ml were inoculated in Millicell small baskets (12mm, 0.4μm, Millicell-PCF, Millipore, USA), and the medium was replaced every other day. When the cells were cultured for 19-25 days, the cells fused to form a dense monolayer, and the EVOMTM epithelial voltage ohmmeter was used to (World Precision Instrument, Sarasota, FL) and only cell monolayers with a TEER greater than 4000 x cm2 were used for transport experiments. At the beginning of the test, the medium in each well was blotted dry, carefully washed twice with 37°C Hank’s solution, 0.4ml was added to the small basket, and 0.6ml blank Hank’s solution was added, and pre-equilibrated at 37°C for 30 minutes. Both the inner and outer solutions of ...
Embodiment 2
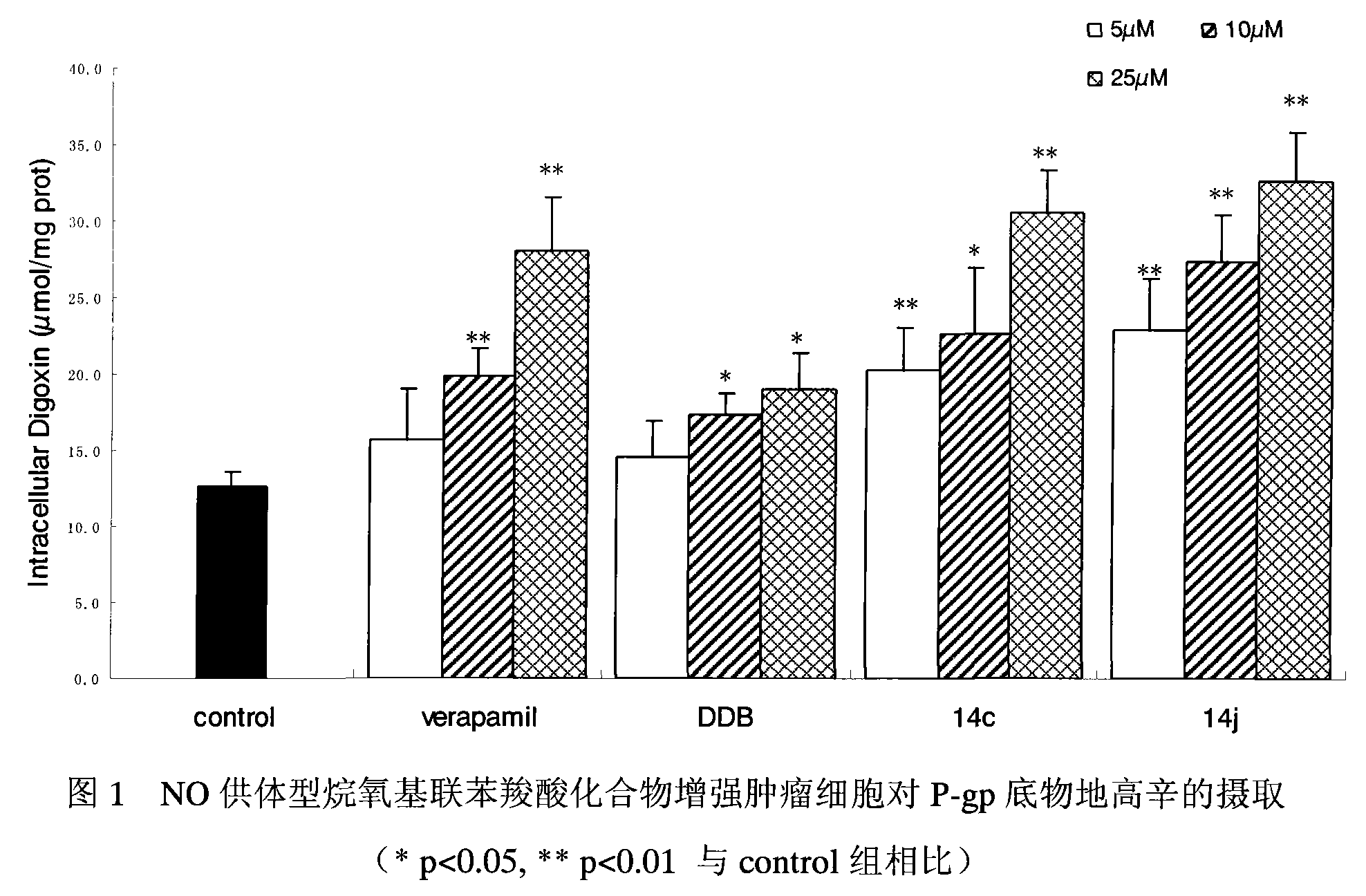
[0039] NO-donating alkoxybiphenylcarboxylic acid compounds enhance the uptake of P-gp substrate digoxin, exemplified by 14c and 14j.
[0040] experimental method:
[0041] Caco-2 cells were seeded on 24-well culture plates, and the culture medium was changed every 3 days or so. When the cell monolayer was completely confluent, the uptake test could be carried out. Before the uptake experiment, first suck out the medium in the 24-well plate, add 37 ℃ blank Hank's solution to the wall to wash the cells for 3 times, gently blot the liquid in the well, add 1ml of the drug solution diluted with Hank's solution, and each drug The final concentrations were 5 μM, 10 μM, and 25 μM, respectively. Place the cell plate in a 37°C incubator and record the time. After 90 minutes, the cell plate was taken out, the solution in the well was sucked out immediately, and 1 ml of digoxin solution diluted with the above-mentioned drug-containing solution was added correspondingly, so that the fina...
Embodiment 3
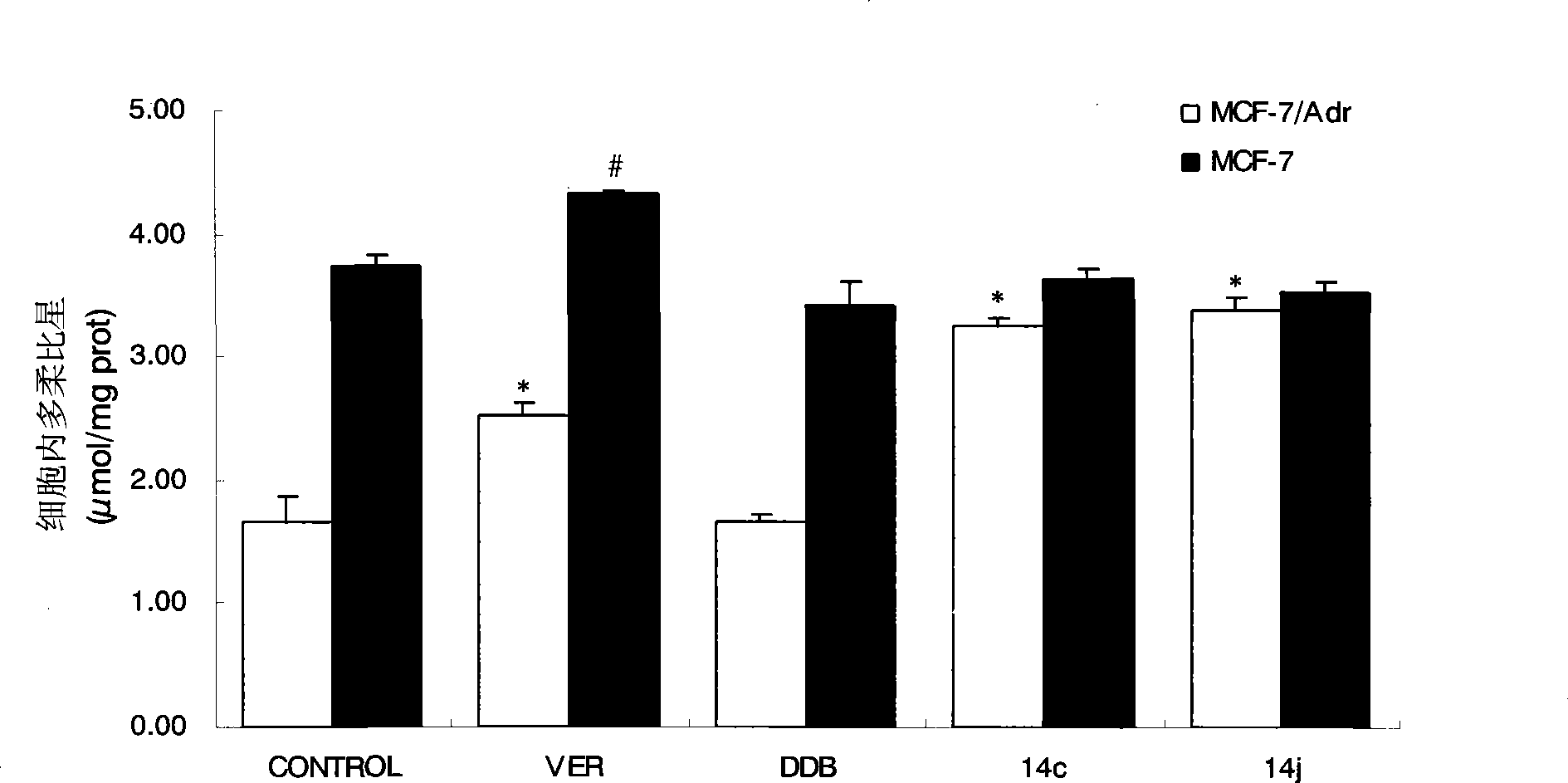
[0044] NO-donating alkoxybiphenylcarboxylic acid compounds enhance the uptake of P-gp substrate doxorubicin by tumor drug-resistant cells, taking 14c and 14j as examples.
[0045] experimental method:
[0046] MCF-7 and MCF-7 / Adr cells were seeded on 24-well culture plates, and the medium was replaced every other day. When the cell monolayer was completely confluent, the uptake test could be performed. Before the uptake experiment, first suck out the medium in the 24-well plate, add 37 ℃ blank Hank's solution to the wall to wash the cells for 3 times, gently blot the liquid in the well, add 1ml of the drug solution diluted with Hank's solution, and each drug The final concentration was 25 μM. Place the cell plate in a 37°C incubator and record the time. Take out the cell plate after 90 minutes, suck out the solution in the well immediately, and add 1 ml of doxorubicin solution diluted with the above-mentioned drug-containing solution correspondingly, so that the final concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com