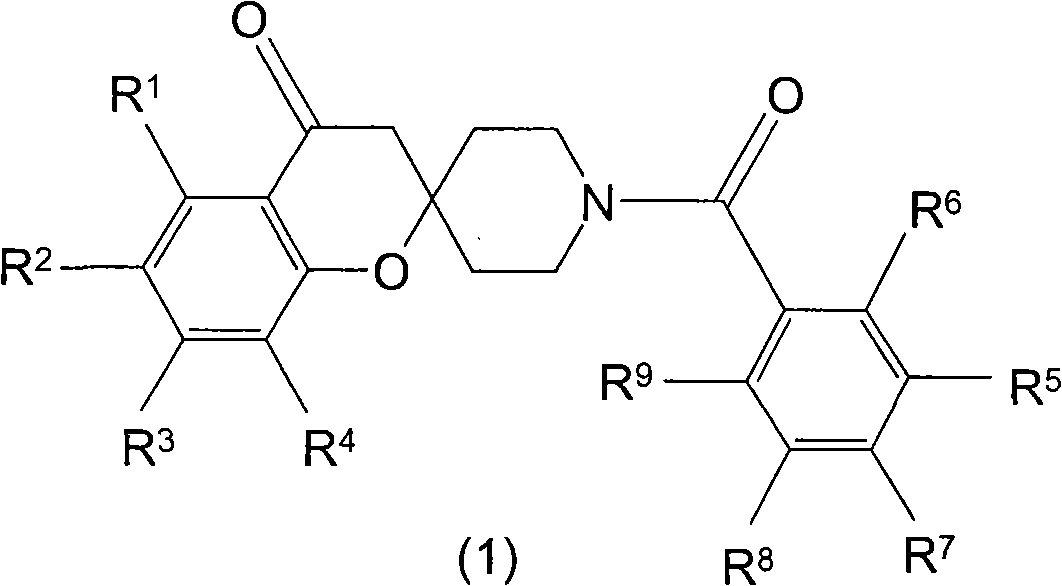
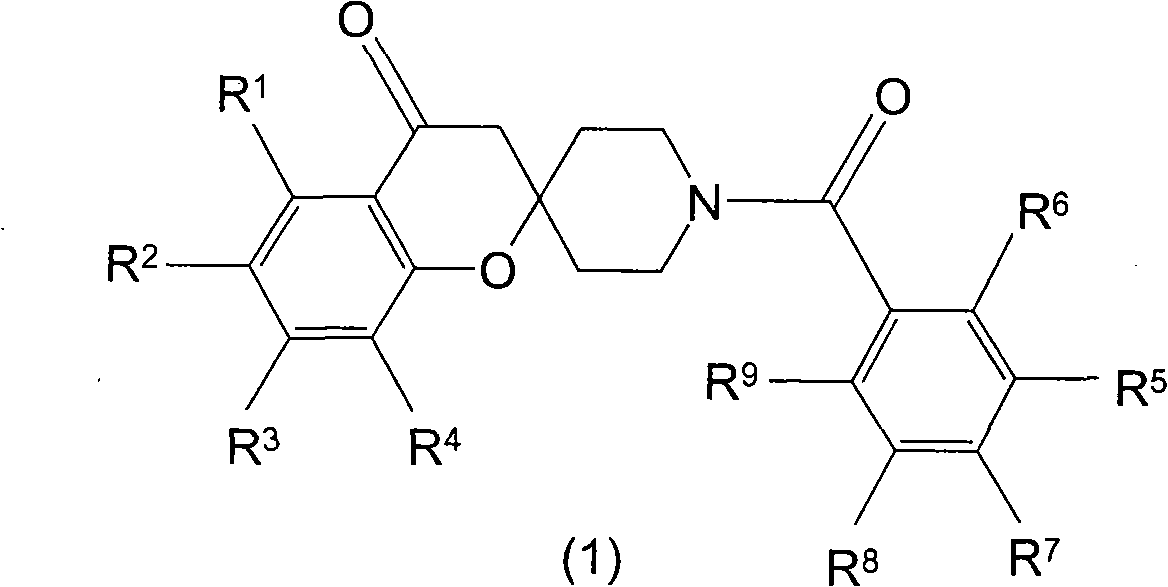
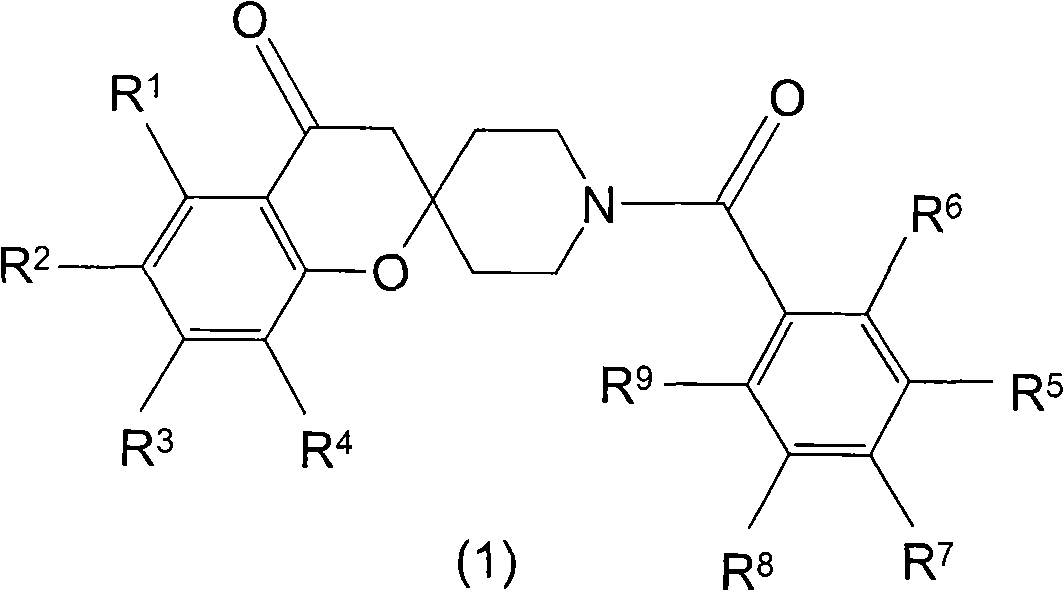
Spiroketone acetyl-CoA carboxylase inhibitors
A technology of alkyl and phenyl, applied in the field of 1'-spiro[benzopyran-2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0091] The examples set forth herein below are for illustrative purposes only. The compositions, methods, and various parameters described herein are merely illustrative of various aspects and embodiments of the invention and are not intended to limit the scope of the invention as claimed in any way.
[0092] All reactants were obtained from commercial sources unless otherwise stated.
[0093] Flash chromatography was performed according to the method described by Still et al., J. Org. Chem., 1978, 43, 2923.
[0094] Use 40M or 40S Biotage with KP-SIL silica (40-63 μM, 60 Å) Columns (Bioatge AB; Uppsala, Sweden) were used for all Biotage described herein purification.
[0095] Fill RediSep with Columns of silica utilizing CombiFlash Companion systems (Teledyne Isco; Lincoln, Nebraska) performed all Combiflashes described herein purification.
[0096] Mass spectra were recorded on a Waters (Waters Corp.; Milford, MA) Micromass Platform II spectrometer. Mass spectra w...
Embodiment B1-B14
[0428]
[0429] Ex.
square
Law
R 1
R 2
R 3
R 4
ACC1
IC 50
(nM)
ACC1
n *
ACC2
IC 50
(nM)
ACC2
n *
MS(ACPI)
m / z
(M+H) +
HPLC
RT
(min)
B1
B
H
CH 3
CH 3
H
24.1
1
424
2.3
B2
B
H
OCH(CH 3 ) 2
H
H
16.1
1
31.1
1
454
2.5
B3
B
H
OCH 2 CH 3
H
H
21.4
1
34.3
1
440
2.4
B4
B
H
Cl
CH 3
H
56.8
2
127
1
444
2.5
B5
B
H
OCH 3
H
H
81.0
1
231
1
426
2.3
B6
B
OCH 3
H
H
H
109
1
426
1.9
B7 ...
Embodiment C1-C5
[0446]
[0447] Ex.
method
R1
R2
R3
R4
ACC1
IC 50
(nM)
ACC1
n *
MS(ACPI)
m / z(M+H) +
HPLC
RT
(min)
C1
B
H
CH 3
CH 3
H
17.5
2
418
2.5
C2
B
H
OCH 3
H
H
22.6
2
420
2.2
[0448] C3
B
OCH 3
H
CH 3
H
29.5
2
434
2.2
C4
B
H
C1
CH 3
H
30.6
2
438
2.7
C5
B
H
CO(O)CH 3
H
H
49.8
2
448
2.3
[0449] * -n is the number of times the trial is performed.
[0450] Ex.C1: 1'-[(7-ethyl-1H-indazol-5-yl)carbonyl]-6,7-dimethylspiro[benzopyran-2,4'-piperidine]- 4(3H)-ketone.
[0451] Ex.C2: 1'-[(7-ethyl-1H-indazol-5-yl)carbonyl]-6-methoxyspir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com