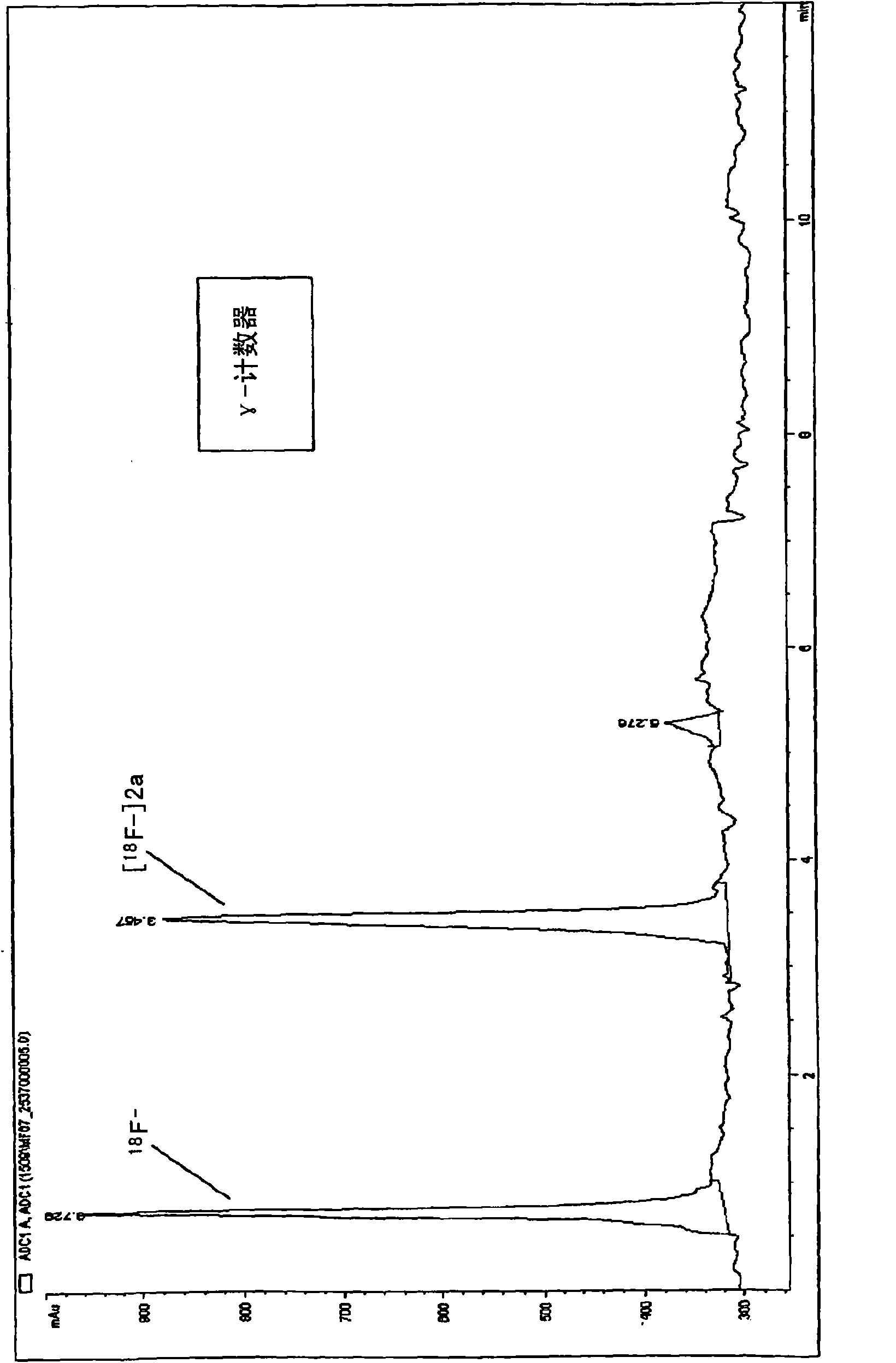
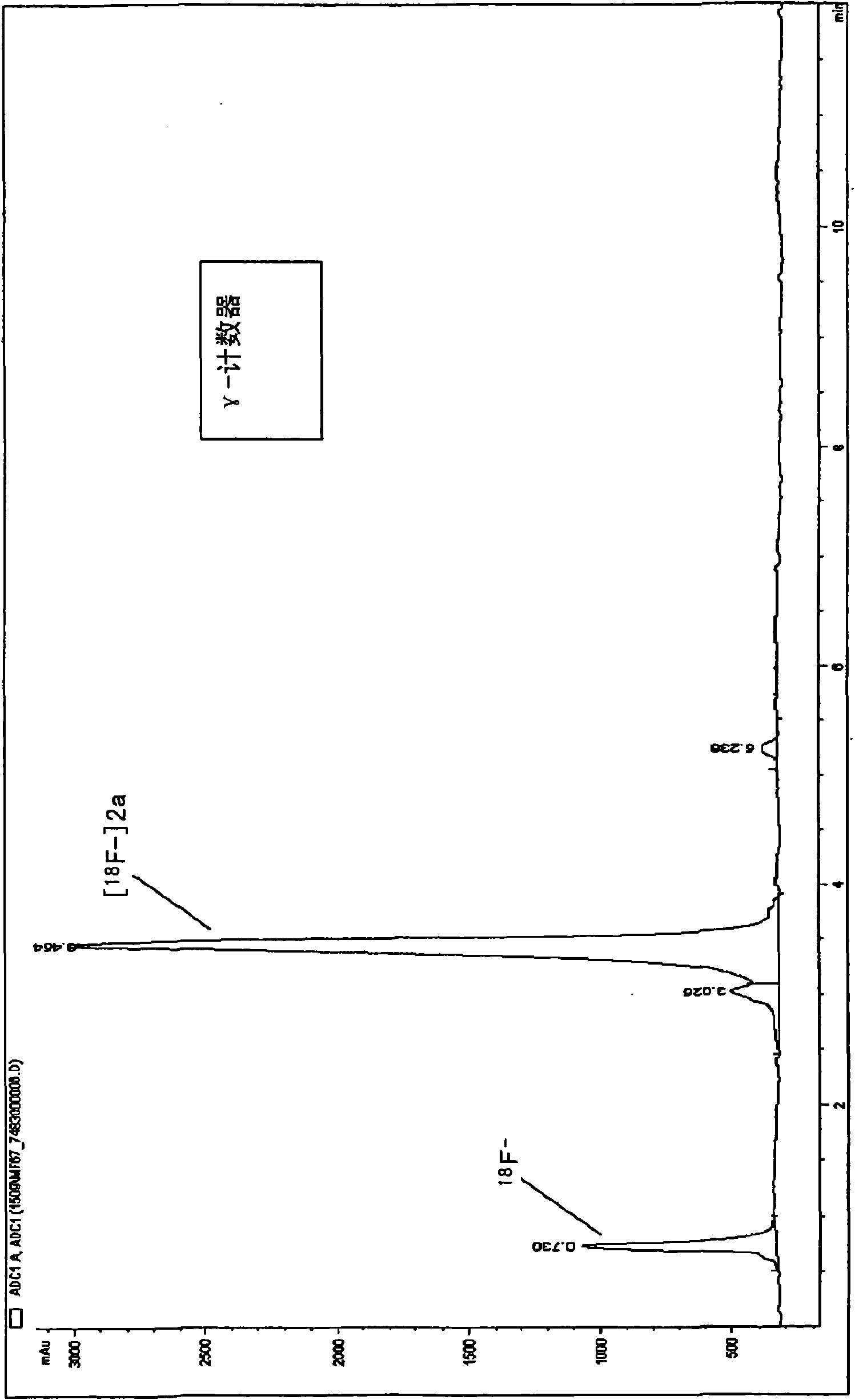
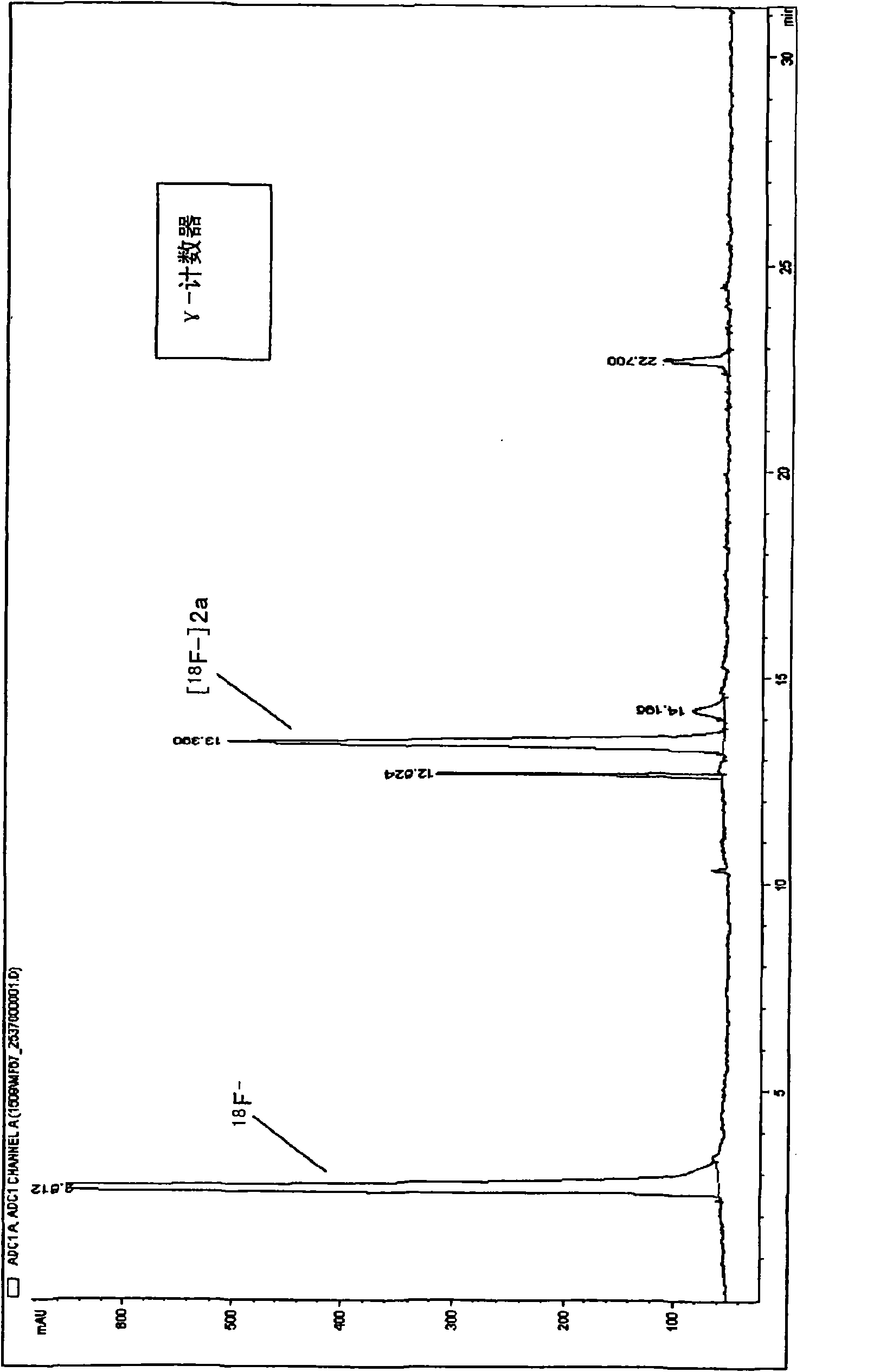
18f fluoro-benzoyl labelled biological active coumpounds as diagnositic imaging agents as well as benzotriazol-1-yloxy-benzoyl, 2,5-dioxo-pyrrolidin-1-yloxy) benzoyl and trimethylammonio-benzoyl precu
A compound and chemical technology, which can be used in the introduction of heterocyclic compound isotopes, NMR/MRI contrast agents, radioactive carriers, etc., and can solve problems such as time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0574] Azeotropically dry by heating at 110-120°C for 20-30 minutes under nitrogen flow in the presence of Kryptofix 222 (5 mg in 1.5 ml MeCN) and cesium carbonate (2.3 mg in 0.5 ml water) 18 F-fluoride (up to 40GBq). During this time, 3 x 1 ml MeCN was added and evaporated. After drying, a solution of the precursor (2 mg) in 150 μl DMSO was added. Seal the reaction vessel and heat at 50-70 °C for 5-15 minutes to achieve labeling. The reaction was cooled to room temperature and diluted with water (2.7ml). The crude reaction mixture was analyzed using analytical HPLC. The product was obtained by preparative radiation HPLC to obtain the desired 18 F labeled peptide.
[0575] In a fourth aspect, the present invention relates to a composition comprising a compound of general chemical formula I or II, more specifically formula IIA or IIB, or a pharmaceutically acceptable salt, hydrate, ester, amide, solvate thereof Or prodrug and further comprising a pharmaceutically acceptab...
Embodiment
[0605] Depends on part LG-O-(C 6 Y 1 Y 2 Y 3 Y 4 )-(??) The properties of the compound of general chemical formula I of the present invention can be synthesized. The peptide moieties of -A-B-D-P are conveniently prepared according to generally established techniques known in the art of peptide synthesis, such as solid phase peptide synthesis. They are a proven Fmoc-solid-phase peptide synthesis with alternating protection and deprotection. These methods are thoroughly discussed in the peptide literature. (Reference: "Fmoc Solid Phase Peptide Synthesis A practical approach", by W.C. Chan and P.D. White, Oxford University Press 2000) (For abbreviations, see Descriptions).
[0606] summary
[0607] Rink amide resin (0,68 mmol / g), carrying out peptide synthesis. If not indicated otherwise, all amino acid residues are L-amino acid residues.
[0608] Fmoc-deprotection (general approach)
[0609]The resin-bound Fmoc peptide was treated with 20% piperidine (v / v) in DMF for...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap