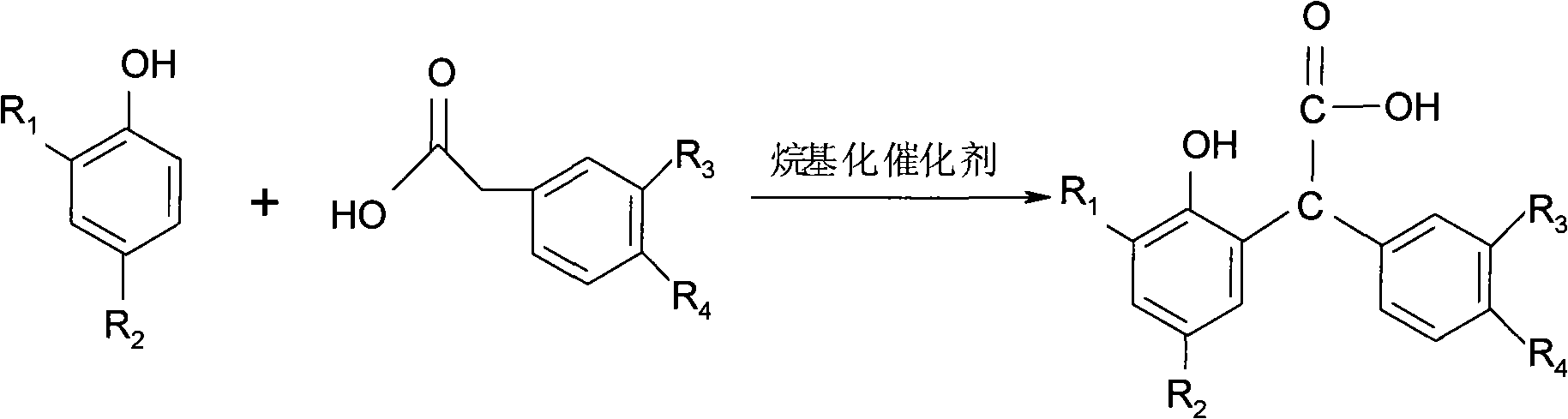
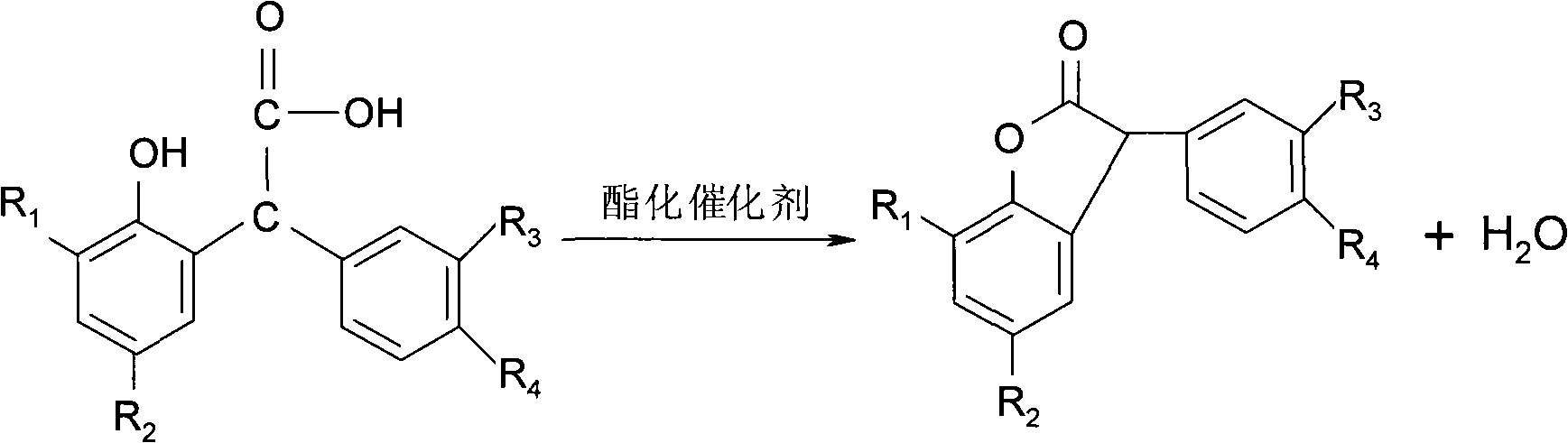
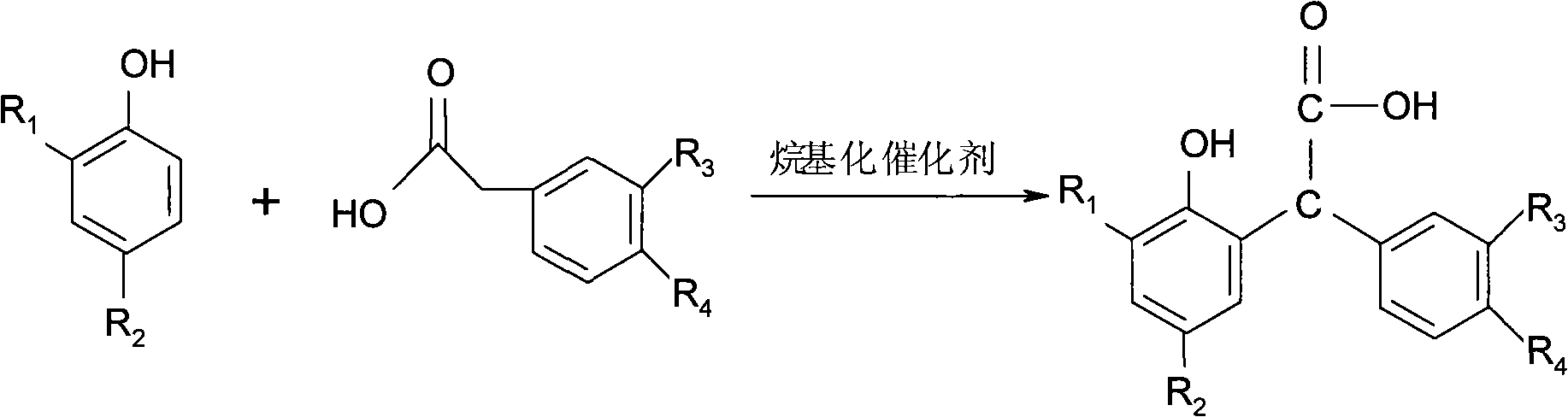
Method for preparing 3-arylbenzofuran ketone compounds
A compound, the technology of furanone, applied in the field of preparation of 3-arylbenzofuranone compound, can solve problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] Preparation of cross-linking agent: hydroxyaluminum polyion [Al 13 o 4 (OH) 24 (H 2 O) 12 ] 7+ (denoted as Al 13 ) preparation: weigh AlCl 3 ·6H 2 O (0.12mol, 28.92g) was added to 600ml of water to make a 0.2mol / L aqueous solution, and 26% NH 3 ·H 2 O solution (0.258mol, 16.8692g), add 1300ml of water to make a 0.2mol / L aqueous solution, add 3 Add NH dropwise to the solution (0.2mol / L) 3 ·H 2 O solution (NH 3 / Al 3+ The molar ratio is 2.15), and then aged in a constant temperature water bath at 70°C for 24 hours to obtain a colorless transparent liquid.
[0038] Acidification, impregnation loading, crosslinking and activation:
[0039] Sodium-based montmorillonite (Na-Mont), first undergoes purification treatments such as grinding, dissolving, and desanding, and then weighs 200 g of purified montmorillonite and places it in 4000 ml of H2O with a mass concentration of 5-50%. 2 SO 4 , HCl, H 3 PO 4 , HAc, HClO 4 In one or more of the solutions, stir and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com