Nitrogenous benzheterocycle derivate and application thereof to treating nervous and mental diseases
A nitrogen heterocycle and derivative technology is applied to benzo nitrogen-containing heterocycle derivatives and their application in the field of drugs for treating neuropsychiatric diseases, and can solve the problems of elevated prolactin, narrow therapeutic spectrum, obvious extrapyramidal symptoms, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
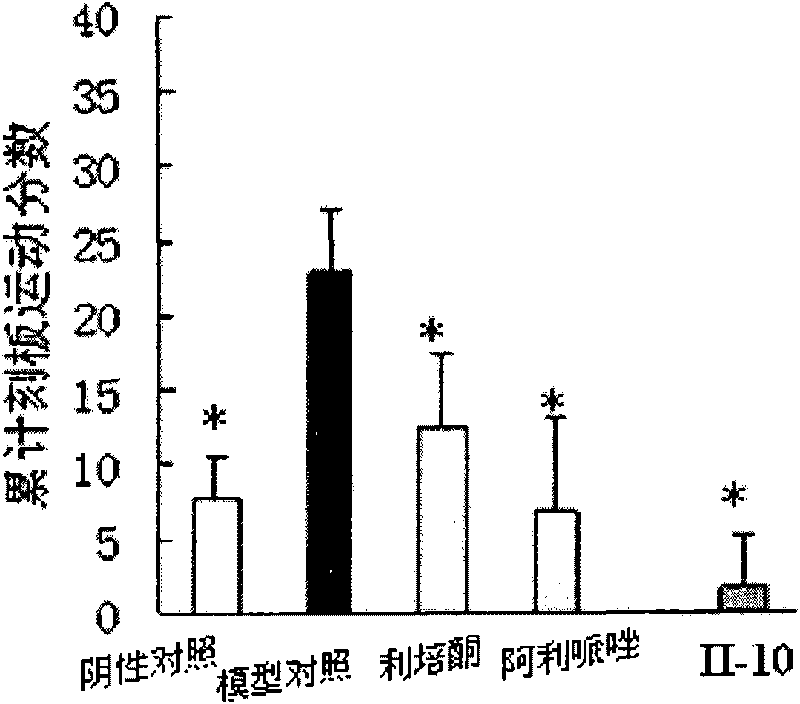
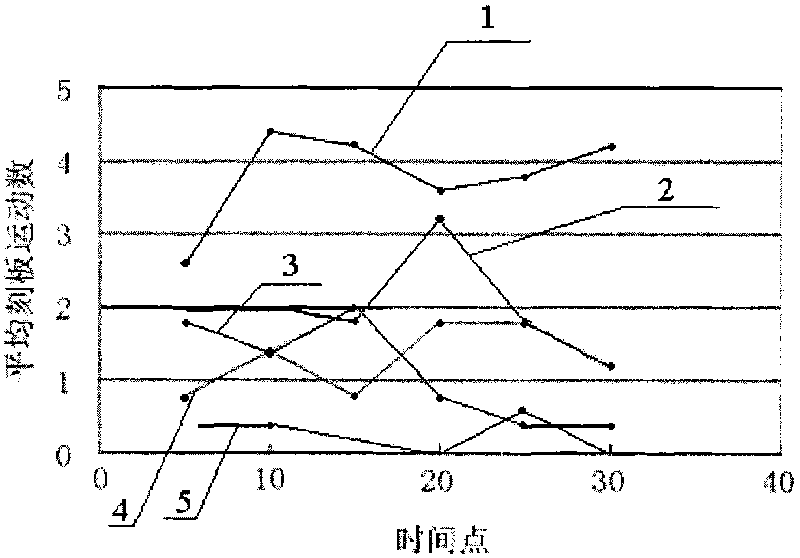
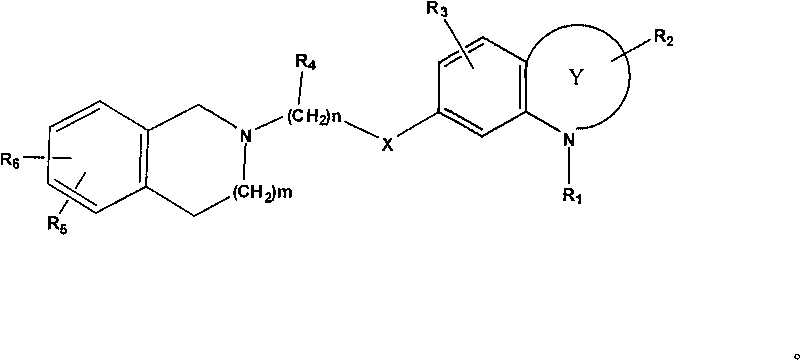
Image
Examples
Embodiment 1
[0099] I-1 7-(4-(3,4-dihydro-2[1H]isoquinolinyl) n-butoxy)-3,4-dihydro-2(1H)quinolone hydrochloride as 1,2 , 3,4-tetrahydroisoquinoline, 1-bromo-4-chloro-butane and 7-hydroxyl-3,4-dihydro-2 (1H) quinolones are raw materials, and 7-( 4-(3,4-dihydro-2[1H]isoquinolinyl)-n-butoxy)-3,4-dihydro-2(1H)quinolone, dissolve the compound in hot absolute ethanol, drop Add hydrochloric acid ethanol to pH = 3, let cool, precipitate white powder, filter, wash with absolute ethanol, and dry at low temperature to obtain the target compound.
[0100] Elemental Analysis: C 22 h 26 N 2 o 2 .HCl.2H 2 O (Theoretical %: C 62.48, H 7.39, N 6.62; Experimental % C62.40, H 7.41, N 6.67)
[0101] 1 HNMR (DMSO-d 6 ): 7.2-7.4(m, 4H, aromatic ring-H), 7.06(d, 1H), 6.51(d, 1H), 6.47(d, 1H), 4.5(d, 1H), 4.3(d, 1H) , 3.95(t, 2H), 3.7(t, 1H), 3.24(m, 3H), 3.11(d, 1H), 2.79(t, 2H), 2.42(t, 2H), 1.90(m, 2H), 1.78 (m, 2H)
[0102] MS: m / e 350.2.
Embodiment 2
[0104] I-2 7-(3-(3,4-dihydro-2[1H]isoquinolinyl)n-propoxy)-3,4-dihydro-2(1H)quinolone hydrochloride
[0105] Using 1,2,3,4-tetrahydroisoquinoline, 1-bromo-3-chloro-propane and 7-hydroxy-3,4-dihydro-2(1H)quinolone as raw materials, prepared according to General Method IV To obtain 7-(3-(3,4-dihydro-2[1H]isoquinolinyl)-n-propoxy)-3,-dihydro-2(1H)quinolone, the compound was dissolved in hot anhydrous In ethanol, add hydrochloric acid ethanol dropwise to pH = 3, let cool, precipitate white powder, filter, wash with absolute ethanol, and dry at low temperature to obtain the target compound.
[0106] Elemental Analysis: C 21 h 24 N 2 o 2 .HCl.H 2 O (Theoretical %C 64.52, H 6.96, N 7.17; Experimental %C64.58, H 7.03, N 7.12)
[0107] 1 HNMR (DMSO-d 6 ): 7.20-7.41(m, 4H), 7.02(d, 1H), 6.51(dd, 1H), 6.47(d, 1H), 4.52(d, 1H), 4.31(d, 1H), 3.95(t, 2H), 3.7(t, 1H), 3.23(m, 3H), 3.11(d, 1H), 2.75(t, 2H), 2.41(t, 2H), 1.90(m, 2H)
[0108] MS: m / e 336.2.
Embodiment 3
[0110] I-3 7-(2-(3,-dihydro-2[1H]isoquinolinyl)ethoxy)-3,4-dihydro-2(1H)quinolone hydrochloride
[0111] Using 1,2,3,4-tetrahydroisoquinoline, 1-bromo-2-chloro-ethane and 7-hydroxy-3,4-dihydro-2(1H)quinolone as raw materials, according to the general method four To obtain 7-(2-(3,4-dihydro-2[1H]isoquinolyl)ethoxy)-3,4-dihydro-2(1H)quinolone, dissolve the compound in hot absolute ethanol , add hydrochloric acid ethanol dropwise to pH = 3, let cool, precipitate white powder, filter, wash with absolute ethanol, and dry at low temperature to obtain the target compound.
[0112] Elemental Analysis: C 20 h 22 N 2 o 2 .HCl (Theoretical %: C 66.94, H 6.46, N 7.81; Experimental %C 66.90H 6.40, N 7.88)
[0113] 1 HNMR (DMSO-d 6 ): 7.15-7.47(m, 4H), 7.03(d, 1H), 6.53(dd, 1H), 6.45(d, 1H), 4.51(d, 1H), 4.30(d, 1H), 3.96(t, 2H), 3.72(t, 1H), 3.24(m, 3H), 3.10(d, 1H), 2.75(t, 2H), 2.41(t, 2H)
[0114] MS: m / e 322.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com