Nitrogenous benzheterocycle derivate and application thereof in treating nervous and mental diseases
A technology of derivatives and nitrogen heterocycles, applied in the field of benzo nitrogen-containing heterocycle derivatives and its application in the treatment of neuropsychiatric diseases, can solve the problems of slow onset of action, obvious extrapyramidal symptoms, elevated prolactin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
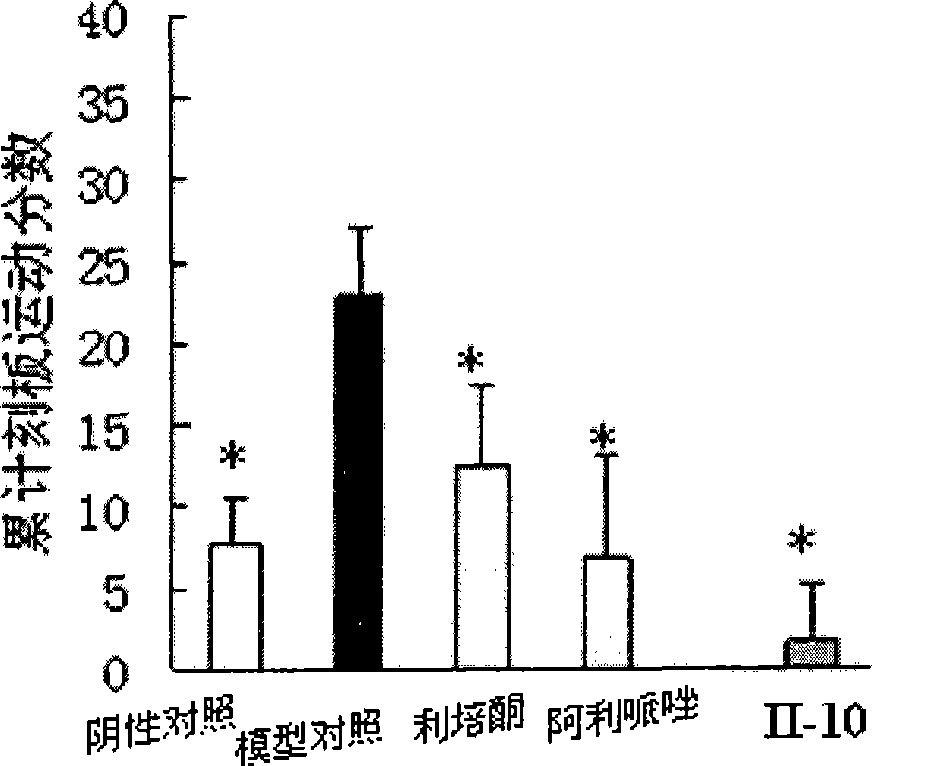
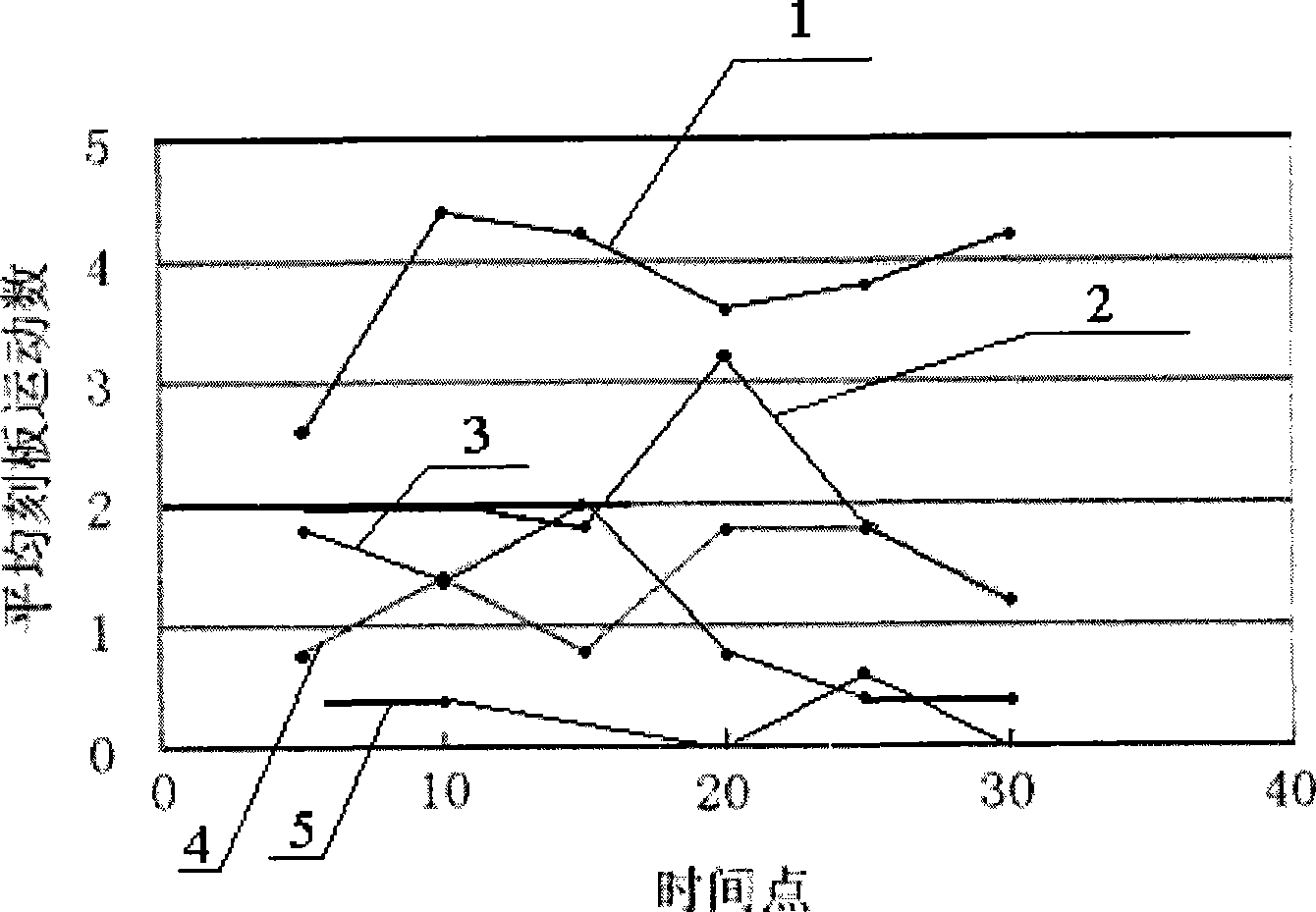
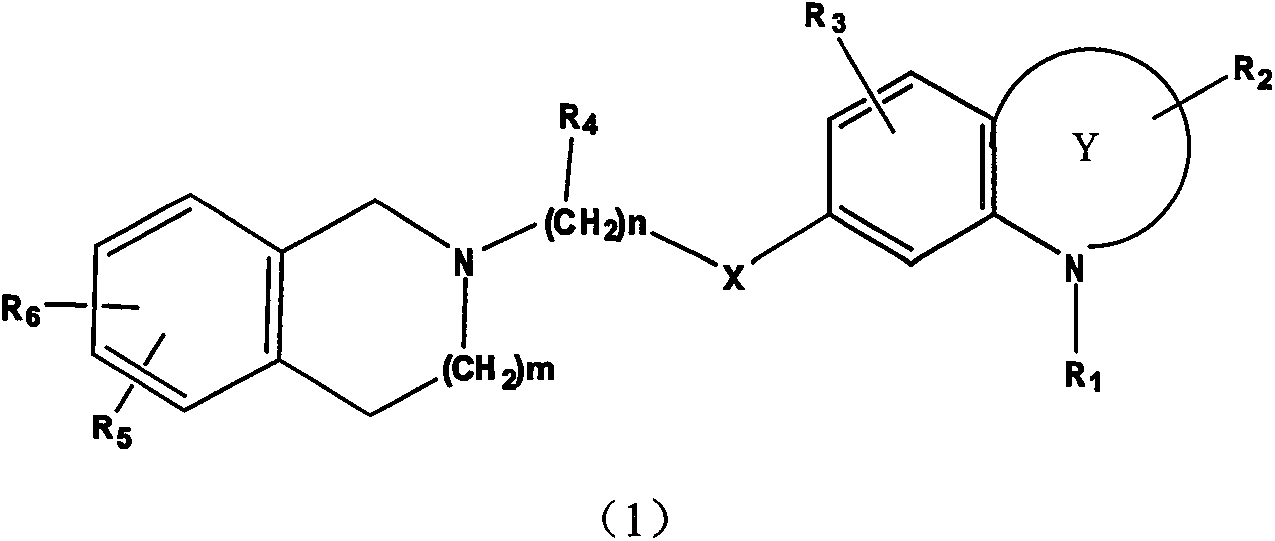
Image
Examples
Embodiment 1
[0099] I-1 7-(4-(3,4-dihydro-2[1H]isoquinolinyl) n-butoxy)-3,4-dihydro-2(1H)quinolone hydrochloride
[0100] Using 1,2,3,4-tetrahydroisoquinoline, 1-bromo-4-chloro-butane and 7-hydroxy-3,4-dihydro-2(1H)quinolone as raw materials, according to the general method four methods Obtain 7-(4-(3,4-dihydro-2[1H]isoquinolinyl) n-butoxy)-3,4-dihydro-2(1H)quinolone, dissolve the compound in hot anhydrous In ethanol, add hydrochloric acid ethanol dropwise to pH = 3, let cool, precipitate white powder, filter, wash with absolute ethanol, and dry at low temperature to obtain the target compound.
[0101] Elemental Analysis: C 22 h 26 N 2 o 2 .HCl.2H 2 O (theoretical value %: C62.48, H7.39, N6.62; experimental value % C62.40, H7.41, N6.67)
[0102] 1 HNMR (DMSO-d 6 ): 7.2-7.4(m, 4H, aromatic ring-H), 7.06(d, 1H), 6.51(d, 1H), 6.47(d, 1H), 4.5(d, 1H), 4.3(d, 1H) , 3.95(t, 2H), 3.7(t, 1H), 3.24(m, 3H), 3.11(d, 1H), 2.79(t, 2H), 2.42(t, 2H), 1.90(m, 2H), 1.78 (m, 2H)
[0103] MS: m / e...
Embodiment 2
[0105] I-2 7-(3-(3,4-dihydro-2[1H]isoquinolinyl)n-propoxy)-3,4-dihydro-2(1H)quinolone hydrochloride
[0106] Using 1,2,3,4-tetrahydroisoquinoline, 1-bromo-3-chloro-propane and 7-hydroxy-3,4-dihydro-2(1H)quinolone as raw materials, prepared according to General Method IV To obtain 7-(3-(3,4-dihydro-2[1H]isoquinolinyl)-n-propoxy)-3,-dihydro-2(1H)quinolone, the compound was dissolved in hot anhydrous In ethanol, add hydrochloric acid ethanol dropwise to pH = 3, let cool, precipitate white powder, filter, wash with absolute ethanol, and dry at low temperature to obtain the target compound.
[0107] Elemental Analysis: C 21 h 24 N 2 o 2 HCl.H 2 O (theoretical value %C64.52, H6.96, N7.17; experimental value %C64.58, H7.03, N7.12)
[0108] 1 HNMR (DMSO-d 6 ): 7.20-7.41(m, 4H), 7.02(d, 1H), 6.51(dd, 1H), 6.47(d, 1H), 4.52(d, 1H), 4.31(d, 1H), 3.95(t, 2H), 3.7(t, 1H), 3.23(m, 3H), 3.11(d, 1H), 2.75(t, 2H), 2.41(t, 2H), 1.90(m, 2H)
[0109] MS: m / e 336.2.
Embodiment 3
[0111] I-3 7-(2-(3,-dihydro-2[1H]isoquinolinyl)ethoxy)-3,4-dihydro-2(1H)quinolone hydrochloride
[0112] Using 1,2,3,4-tetrahydroisoquinoline, 1-bromo-2-chloro-ethane and 7-hydroxy-3,4-dihydro-2(1H)quinolone as raw materials, according to the general method four To obtain 7-(2-(3,4-dihydro-2[1H]isoquinolyl)ethoxy)-3,4-dihydro-2(1H)quinolone, dissolve the compound in hot absolute ethanol , add hydrochloric acid ethanol dropwise to pH = 3, let cool, and precipitate white powder, filter, wash with absolute ethanol, and dry at low temperature to obtain the target compound.
[0113] Elemental Analysis: C 20 h 22 N 2 o 2 HCl (Theoretical %: C66.94, H6.46, N7.81; Experimental % C66.90H6.40, N7.88)
[0114] 1 HNMR (DMSO-d 6 ): 7.15-7.47(m, 4H), 7.03(d, 1H), 6.53(dd, 1H), 6.45(d, 1H), 4.51(d, 1H), 4.30(d, 1H), 3.96(t, 2H), 3.72(t, 1H), 3.24(m, 3H), 3.10(d, 1H), 2.75(t, 2H), 2.41(t, 2H)
[0115] MS: m / e 322.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com