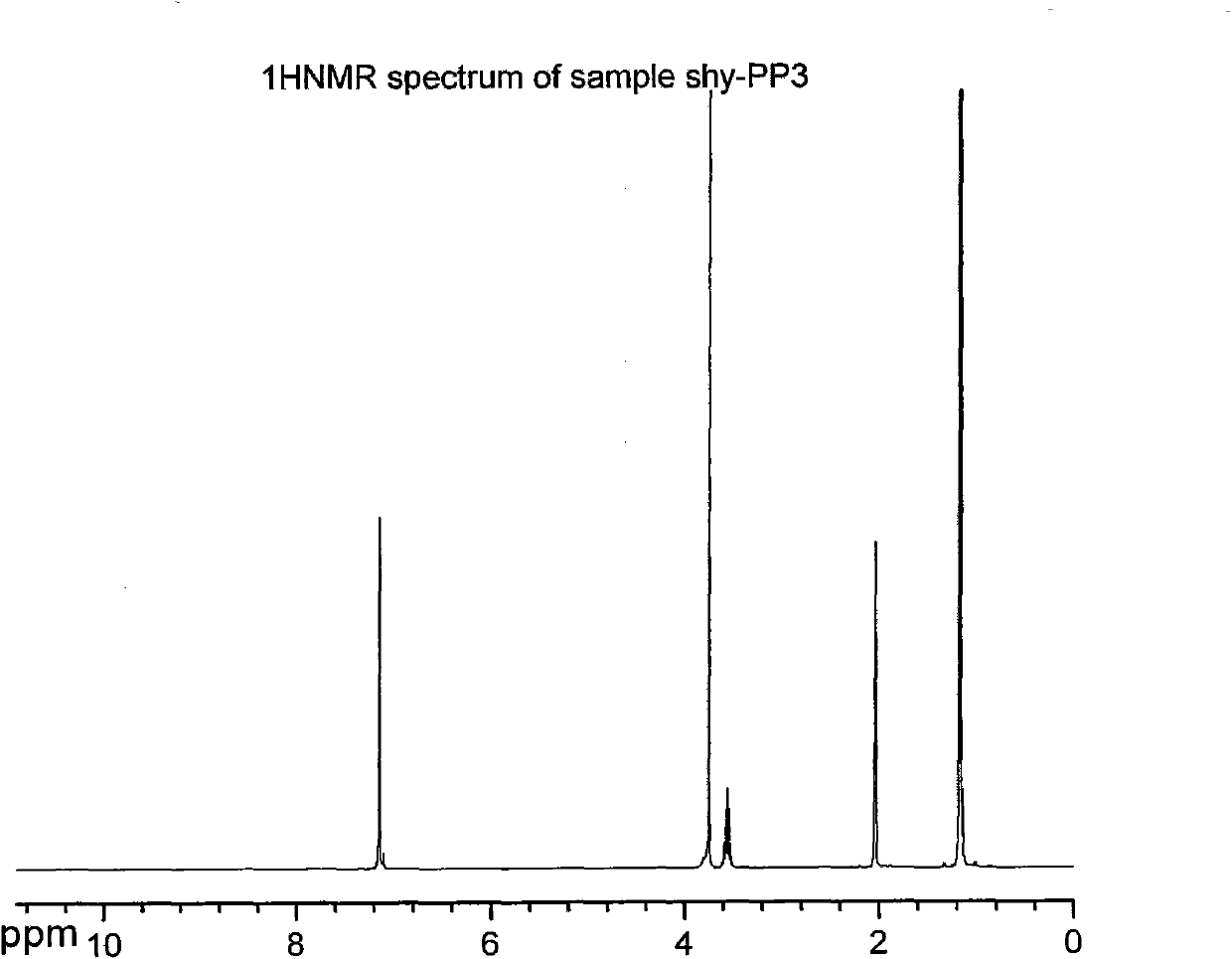
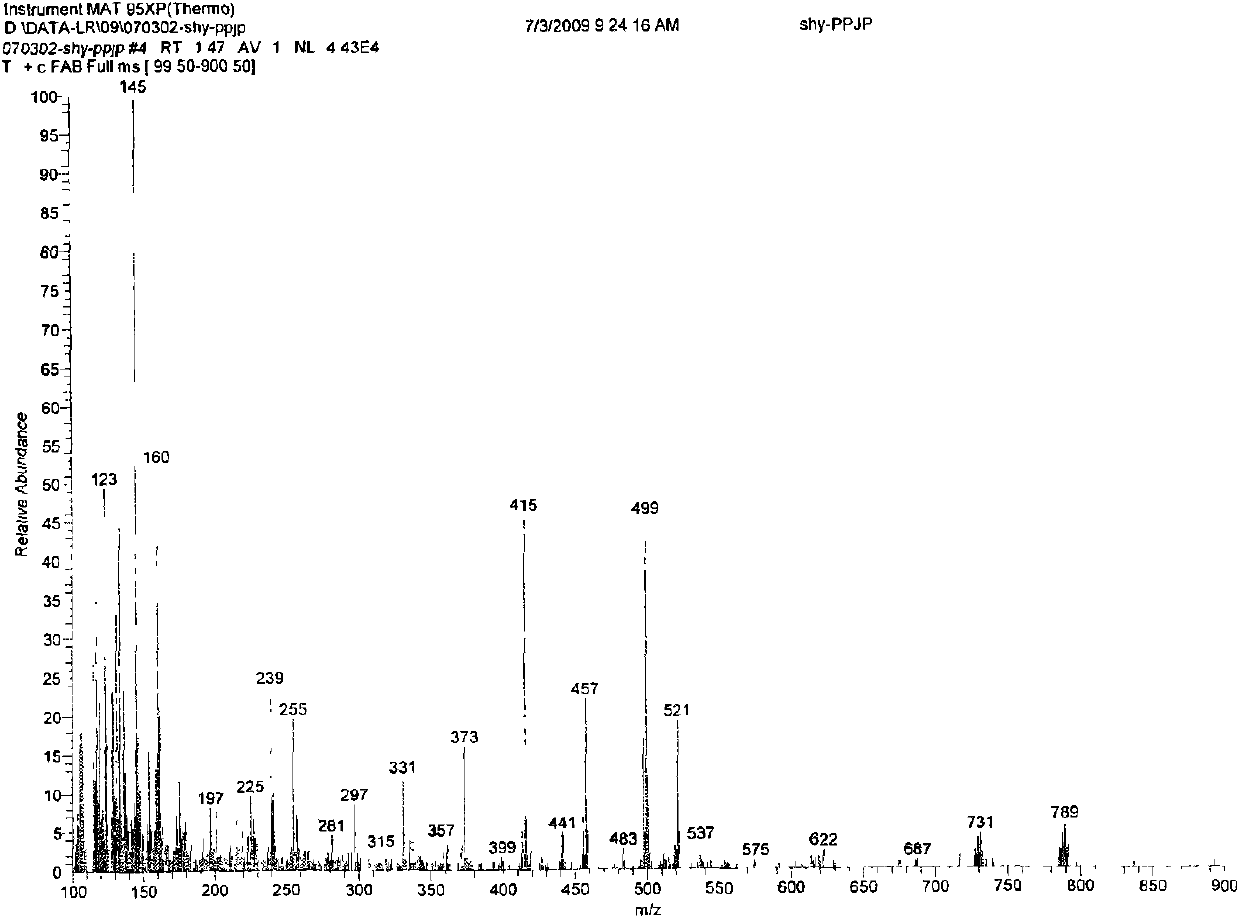
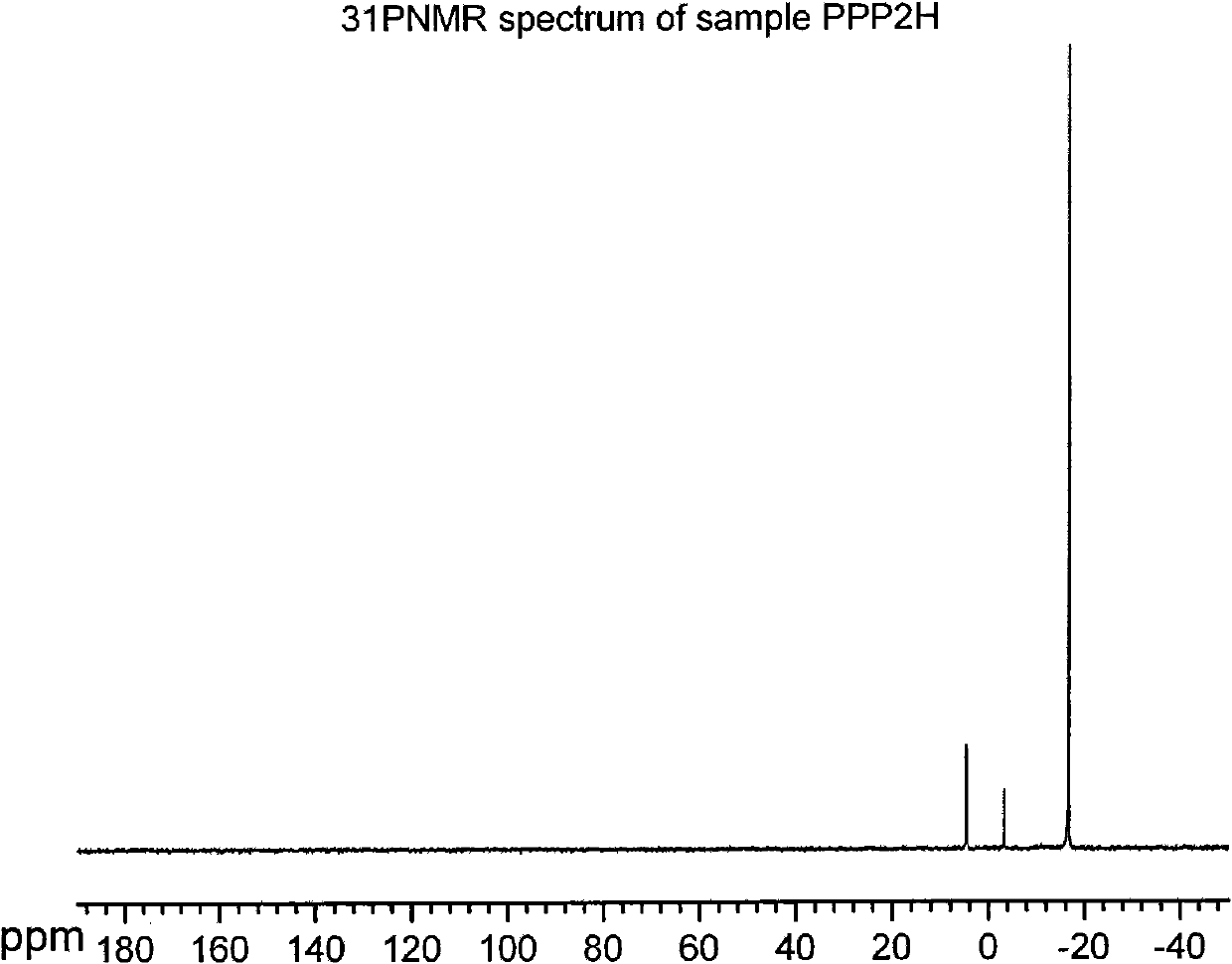
Dipropofol dihydrogen pyrophosphate and salt thereof, preparation method and application thereof
A technology of propofol dihydrogen pyrophosphate and propofol pyrophosphate, which is applied in the preparation of water-soluble prodrug dipropofol dihydrogen pyrophosphate and its salt, and preparation of sedative, hypnotic and anesthetic drugs It can solve the problems of unfavorable drug safety and other problems, and achieve the effects of easy large-scale preparation, high yield and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1.42g (0.01mol) P 2 o 5and 10ml of anhydrous toluene were added to the reaction flask, heated to 80°C under stirring, and 1.78g (0.01mol) of propofol was added dropwise rapidly. Part of the solvent was dried, and the rest was allowed to stand for cooling. A large amount of white solids were formed. Suction filtration and washing with anhydrous toluene gave 1.86 g of white crystals of dipropofol dihydrogen pyrophosphate, with a yield of 74.69%.
Embodiment 2
[0053] 0.71g (0.005mol) P 2 o 5 and 10ml of anhydrous toluene were added to the reaction flask, and under stirring at room temperature, 1.78g (0.01mol) propofol was quickly added dropwise, and the constant temperature was continued to stir and react for 2 hours after the addition was completed. After standing for cooling, a white solid was formed, which was filtered by suction and washed with anhydrous toluene to obtain 1.17 g of white crystals of dihydrogen pyrophosphate of dipropofol, with a yield of 46.98%.
Embodiment 3
[0055] 11.36g (0.08mol) P 2 o 5 and 50ml of anhydrous toluene were added to the reaction flask, heated to 110°C under stirring, and 1.78g (0.01mol) propofol was quickly added dropwise. Part of the solvent was dried, and the remaining part was allowed to stand for cooling, and a white solid was formed, which was filtered by suction and washed with anhydrous toluene to obtain 1.62 g of white crystals of dipropofol dihydrogen pyrophosphate, with a yield of 65.06%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com