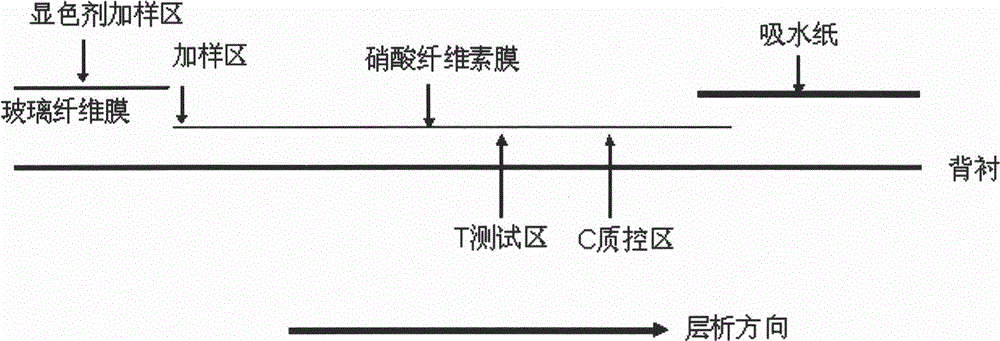
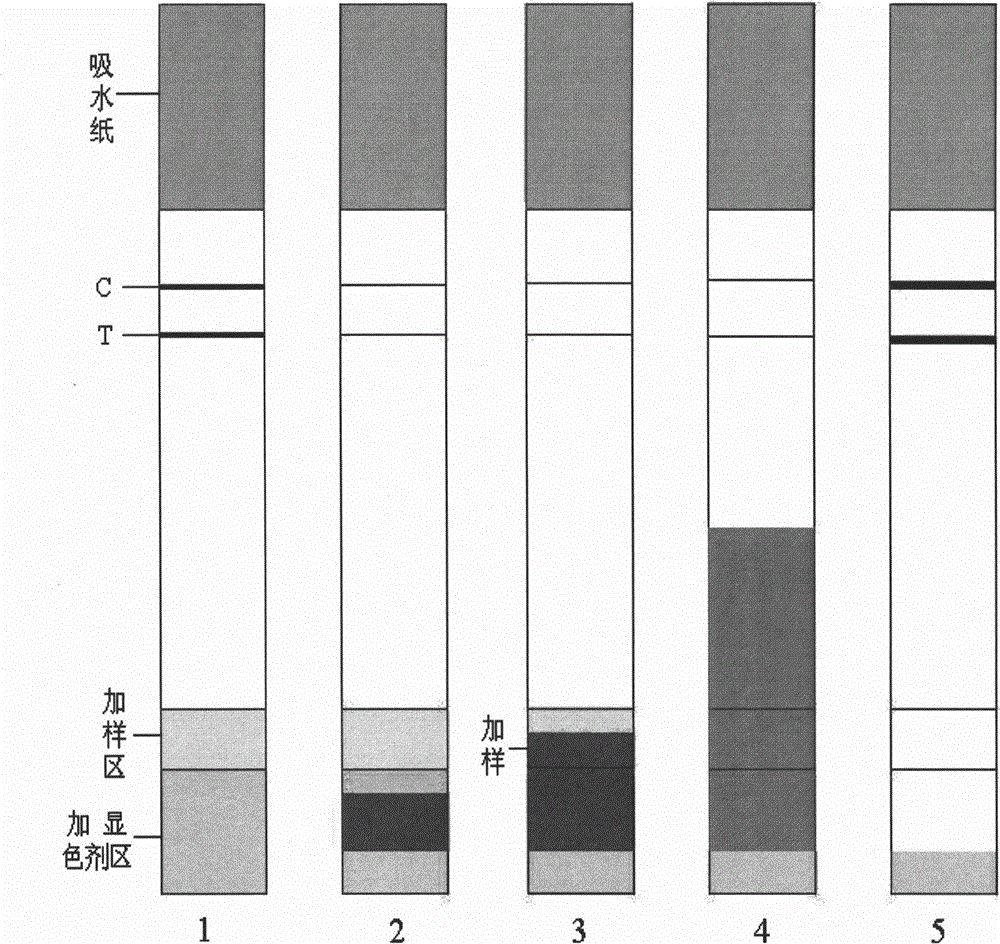
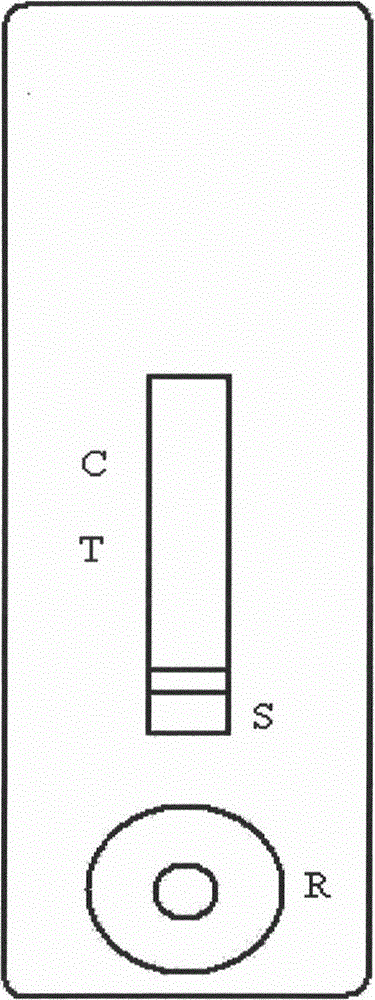
Immunochromatography assay and device taking color-developing agent as sample carrier and capable of repeated sample adding
An immunochromatography and color-developing reagent technology, applied in the field of immunochromatography, can solve the problems of decreased effective utilization rate, long time required to flow through the reaction zone, slow color reaction, etc., and achieves improved sensitivity and shortened interpretation time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 former method and method of the present invention detect HCV (hepatitis C virus) antibody
[0035] In this example, the same amount of samples and the same reagent strips, both 5 mm wide, were used to make them according to the general method, and the original method was compared with the method of the present invention to detect HCV antibodies.
[0036] Among them, the quality control area was coated with 1 mg / ml SPA (recombinant Staphylococcus aureus protein A), with an amount of 0.5 μl / reagent strip, and the coating was linear. Coat the test area with 0.5 mg / ml of HCV antigen, with an amount of 0.5 μl / reagent strip, and the coating is linear. Chromogen mixture: colloidal gold liquid preparation combined with SPA (volume ratio of 1 mg / ml SPA: colloidal gold is 1:500).
[0037]The samples used in the original method and the method of the present invention are both 5 μl HCV critically positive samples (concentration is about 0.00001 mg / ml), and the colloid...
Embodiment 2
[0039] Embodiment 2 The embodiment of repeatable sample addition
[0040] In this embodiment, continue to take the test of HCV as an example, and its reagent strip, test area, quality control area, chromogenic agent mixture (colloidal gold preparation), reagent strip width, and sample are the same as in Example 1.
[0041] The original method: add 5 μl HCV critical sample (concentration is about 0.00001mg / ml) on the nitrocellulose membrane, after the sample runs through the test area, add 100 μl colloidal gold preparation on the glass fiber. After 1 minute, 5 μl of sample was added, and then every 1 minute, 5 μl of sample was added, and repeated twice, that is, a total of 20 μl of sample was added. A total of 30 minutes to interpret the results.
[0042] The method of the present invention: add 100 μ l of colloidal gold preparation on the glass fiber, wait for the colloidal gold to run out of the glass fiber, enter the sample adding area, add 5 μ l of HCV critical sample (con...
Embodiment 3
[0044] Embodiment 3 Fluorescence test HIV (HIV)
[0045] Using the same amount of samples, the original method and the method of the present invention are compared to detect HIV antibodies at the same time. Among them, the reagent strips are the same, made according to the general method, and the quality control area is coated with 1 mg / ml SPA (recombinant Staphylococcus aureus protein A), with an amount of 0.5 μl / strip. The test area is coated with 0.1 mg / ml HCV antigen, and the dosage is 0.5 μl / strip. The width of the reagent strip is 5mm. Chromogenic reagent mixture: a preparation of fluorescein isothiocyanate (FITC) combined with SPA (volume ratio of 1 mg / ml SPA:FITC is 1:500) was used.
[0046] The original method: Add 5 μl HIV critical sample (concentration is about 0.000005mg / ml) on the nitrocellulose membrane, after the sample runs through the test area, add 100 μl FITC (fluorescein isothiocyanate) preparation on the glass fiber. Timed for 15 minutes, and read the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com