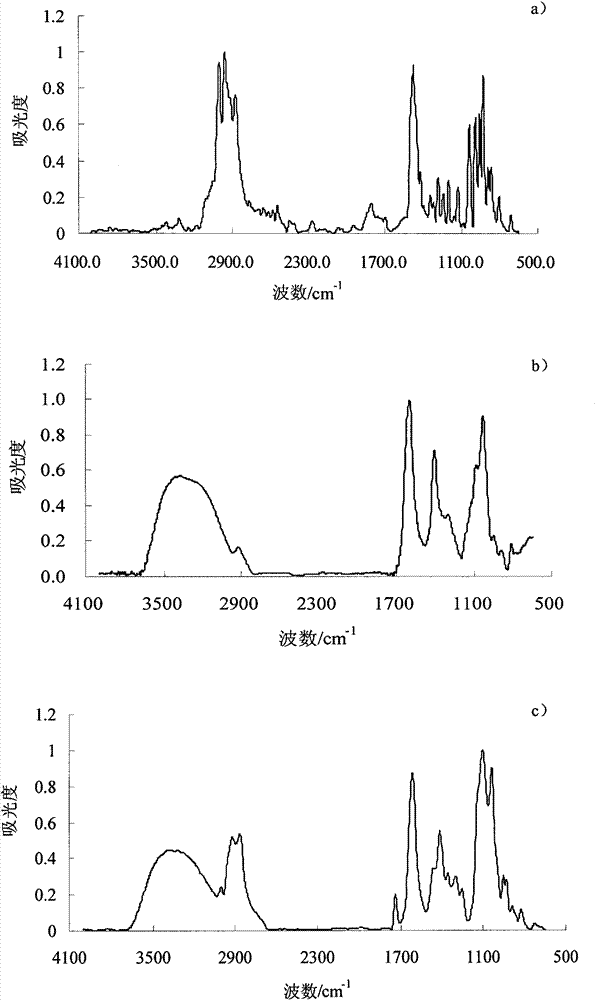
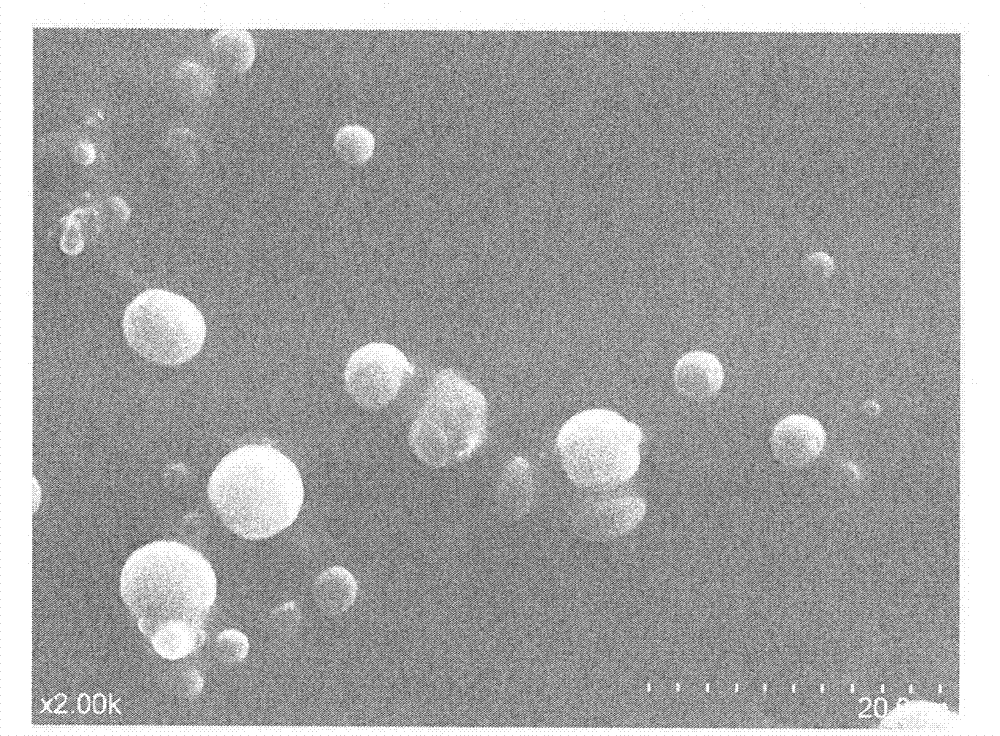
N,N-dimethyl piper-ridinium chloride sustained-release microcapsules and preparation method thereof
A technology of methylpiperium and microcapsules, which is applied in the field of agriculture and can solve the problems of fast release of active ingredients, ecological damage, and short duration of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1, average particle diameter 60 μ m methylpiperium sustained-release microcapsules
[0025] The original drug selects 97.0% methylpiperium original powder produced by Jiangnan Pesticide Factory in Changzhou City, Jiangsu Province (the same as in the following examples).
[0026] Take by weighing 0.4g chitosan and 0.5g methylpiperium and dissolve in 20mL mass concentration and be 2% acetic acid solution, obtain mixed solution; Slowly add 1.0mL castor oil polyoxyethylene ether under the conditions, stir and emulsify for 30min, until the mixture is evenly dispersed; set the inlet temperature of the spray dryer to 150°C, and the outlet temperature to 95°C, and use the spray dryer to solidify and dry the resulting mixture. The obtained powder is mepiperium sustained-release microcapsules.
[0027] The prepared methylpiperium sustained-release microcapsules were observed with an electron microscope (Shimadzu S-3400 type) and their morphology was...
Embodiment 2
[0032] Embodiment 2, the preparation of mepiperium sustained-release microcapsules with an average particle diameter of 20 μm
[0033] Weigh 1.0g sodium alginate, 0.5g gelatin and 1.0g methylpiperium respectively and dissolve them in 100mL water to obtain a mixed solution; Slowly add 1.5mL tristyrylphenol polyoxyethylene polyoxypropylene ether, stir and emulsify for 40min until the mixture is uniformly dispersed; set the inlet temperature of the spray dryer to 145°C, and the outlet temperature to 85°C, and use the spray dryer to dissolve the resulting mixture Carry out curing and drying, and the obtained powder is mepiperium sustained-release microcapsules.
[0034] The method for measuring the performance index of the mepiperium sustained-release microcapsules prepared in this embodiment is the same as that in Example 1. The specific measurement results are: the cyst formation rate of methylpiperium is 83%, the drug loading rate is 21.3%, the average particle size is 19.1 μm...
Embodiment 3
[0036] Embodiment 3, preparation of mepiperium sustained-release microcapsules with an average particle diameter of 3 μm
[0037]Weigh 0.4g chitosan, 0.1g gelatin and 0.5g methylpiperium respectively and dissolve them in 20mL mass concentration of 2% acetic acid solution to obtain a mixed solution; Stir, and slowly add 0.8mL sorbitan monooleate polyoxyethylene ether under stirring conditions, stir and emulsify for 40min, until the mixture is evenly dispersed; set the inlet temperature to 125°C, the outlet temperature to 80°C, The obtained mixed liquid is solidified and dried, and the obtained powder is mepiperium sustained-release microcapsules.
[0038] The method for measuring the performance index of the mepiperium sustained-release microcapsules prepared in this embodiment is the same as that in Example 1. The specific measurement results are: the cyst formation rate of methylpiperium is 75%, the drug loading rate is 25.8%, the average particle size is 2.8 μm, and the rel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com