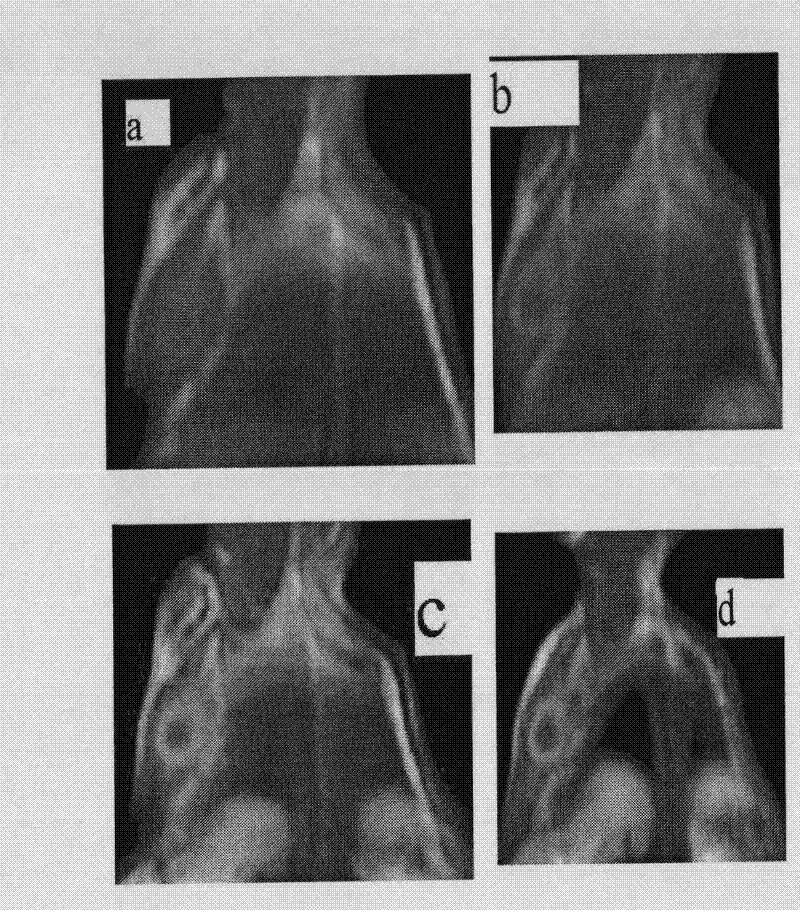
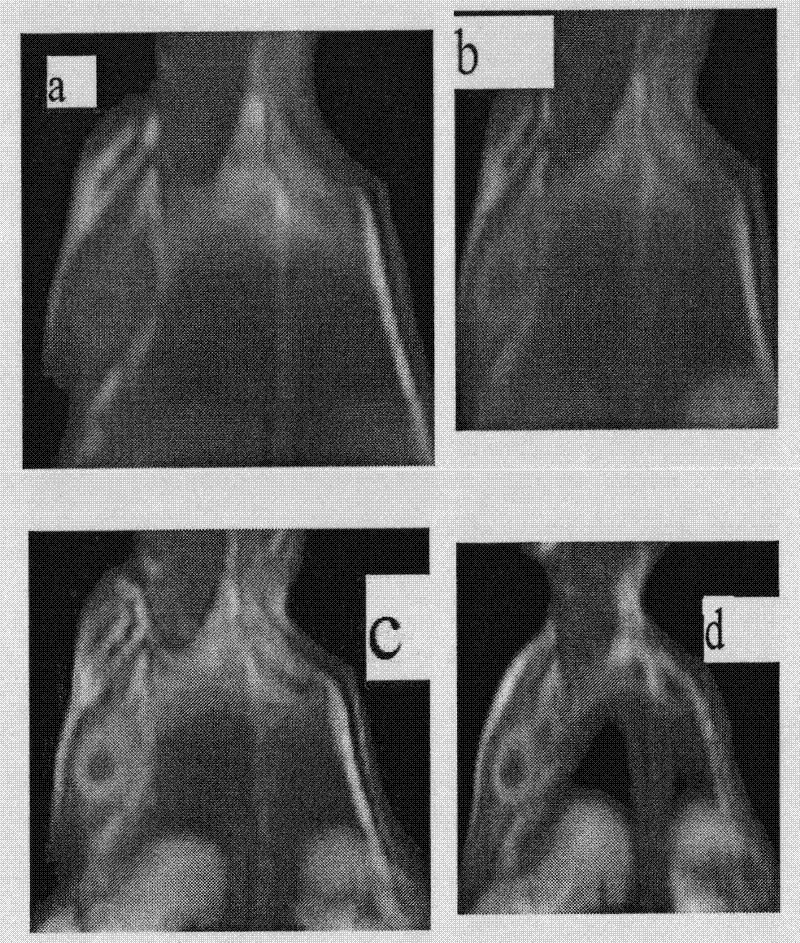
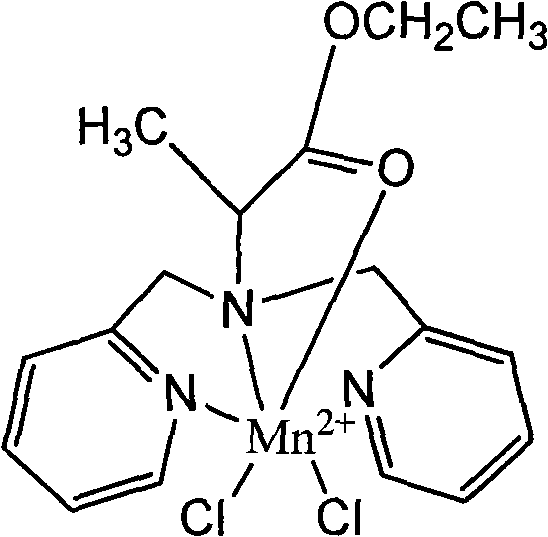
Manganese compound and preparation method and application thereof
A technology of manganese complexes and ligands, applied in the field of imaging agents for magnetic resonance imaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 (best reaction condition example):
[0021] The ligand N-(2-propionyl ethyl)-N,N-bis(2-pyridylmethyl)amine and MnCl 2 Dissolve in an aqueous solution at a molar ratio of 1:1 and control at 30°C; add ammonium chloride (0.1 mol) as a catalyst, and the reaction time is 2 hours. The product was purified by silica gel column chromatography (developing solvent: tetrahydrofuran:petroleum ether=3:2), and concentrated to remove the solution to obtain a light yellow manganese complex: [LMnCl 2 ]. Yield, 63%. Molecular formula: C 17 h 21 Cl 2 MnN 3 o 2 Elemental analysis: measured value C, 48.34; H, 4.62; N, 9.58, Mn, 12.62. Calculated value C, 48.02; H, 4.98; N, 9.88, Mn, 12.92.IR(v cm -1 , KBr): 3057m (=CH), 2986m (-CH 2 -), 1730m(C=O), 1603s, 1571m, 1442s, 777s(pyridine).UV-vis((H 2 O / nm, vcm -1 ,ε)38461(8900).
Embodiment 2
[0023] The ligand N-(2-propionyl ethyl)-N,N-bis(2-pyridylmethyl)amine and MnCl 2 Dissolve in aqueous solution at a molar ratio of 2:1, controlled at 20°C; add ammonium chloride (0.1 mol) as a catalyst, and react for 1 hour. The product was purified by silica gel column chromatography (developing solvent: tetrahydrofuran:petroleum ether=3:2), and concentrated to remove the solution to obtain a light yellow manganese complex: [LMnCl 2 ]. Yield: 25%. Molecular formula: C 17 h 21 Cl 2 MnN 3 o 2 Elemental analysis: measured value C, 48.34; H, 4.62; N, 9.58, Mn, 12.62. Calculated value C, 48.02; H, 4.98; N, 9.88, Mn, 12.92.IR (v cm -1 , KBr): 3057m (=CH), 2986m (-CH 2 -), 1730m(C=O), 1603s, 1571m, 1442s, 777s(pyridine).UV-vis((H2 O / nm, vcm -1 ,ε)38461(8900).
Embodiment 3
[0025] The ligand N-(2-propionyl ethyl)-N,N-bis(2-pyridylmethyl)amine and MnCl 2 Dissolve in an aqueous solution at a molar ratio of 1:1, and control at 40°C; add ammonium chloride (0.1 mol) as a catalyst, and react for 1 hour. The product was purified by silica gel column chromatography (developing solvent: tetrahydrofuran:petroleum ether=3:2), and concentrated to remove the solution to obtain a light yellow manganese complex: [LMnCl 2 ]. Yield: 45%. Molecular formula: C 17 h 21 Cl 2 MnN 3 o 2 Elemental analysis: measured value C, 48.34; H, 4.62; N, 9.58, Mn, 12.62. Calculated value C, 48.02; H, 4.98; N, 9.88, Mn, 12.92.IR (v cm -1 , KBr): 3057m (=CH), 2986m (-CH 2 -), 1730m(C=O), 1603s, 1571m, 1442s, 777s(pyridine).UV-vis((H 2 O / nm, vcm -1 ,ε)38461(8900).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com