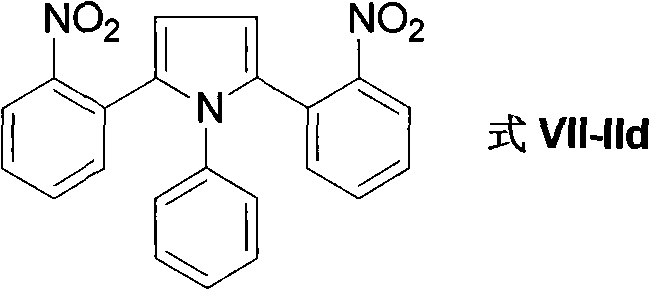
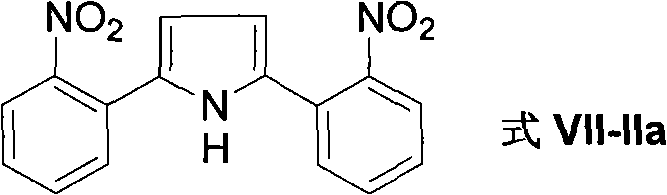
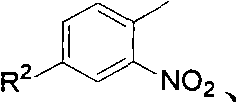
Method for preparing 2-(2-nitrophenyl) pyrrole and 2,5-bis(2-nitrophenyl) pyrrole compounds
A kind of technology of compound of general formula, phenyl, applied in the field of preparing 2-pyrroles and 2, to achieve the effect of expanding the scope of application
Inactive Publication Date: 2010-09-01
胡跃飞
View PDF1 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
But this method needs pyrrole compound to be prepared into magnesium salt or zinc salt in advance, or needs to use higher reaction temperature and expensive metal catalyst, but can only obtain medium yield, so be subject to certain restriction when using [references: (a) Miura, M.; Nomura, M. Top. Curr. Chem. 2002, 219, 211-241; (b) Proch, S.; Kempe, R. Angew. 3135-3138; (c) Stuart, D.R.; Fagnou, K.Science.2007, 316, 1172-1175; (d) Hull, K.L.; Sanford, M.S.J.Am.Chem.Soc.2007, 129, 11904-11905]
Directly introducing aryl substituents on heterocyclic rings through nucleophilic aryl substitution reactions is also a convenient method for the synthesis of aryl-substituted heterocyclic compounds, but this method has not yet been used to introduce aryl substituents at the 2-position of pyrrole compounds. [Partial references: (a) Bunnett, J.F.; Zahler, R.E.Chem.Rev.1951, 49, 273-412; (b) Shi, Y.J.; Humphrey, G.et al.Adv.Synth.Catal. 2006, 348, 309-312; (c) Ueno, M.; Yonemoto, M. et al. Chem. Commun. 2007, 2264-2266]; and if a pyrrole compound without an N-protecting group is used as a reactant , the reaction takes place on the N-atom, not on the C-atom on the pyrrole ring
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a method for preparing 2-(2-nitrophenyl) pyrrole and 2,5-bis(2-nitrophenyl) pyrrole compounds. In the method, a compound shown in a general formula V and a compound shown in a general formula VI undergo reaction similar to nucleophilic aromatic substitution reaction under the alkali condition to obtain a compound shown in a general formula VII in a mode of high efficiency and high regioselectivity. The reaction has high regioselectivity; even if an N-H pyrrole compound or a pyrrole compound with an N-H substituent group is used as a substrate, the aromatic substitution reaction can still be performed at 2-position and 5-position of the pyrrole compound to obtain a C-arylation product in a mode of high regioselectivity; and no N-arylation product is generated. Thus, the characteristic ensures that the reaction can be used in the pyrrole compound which contains activated hydrogen, thereby expanding the application scope of the method.
Description
technical field The invention relates to a method for preparing 2-(2-nitrophenyl)pyrroles and 2,5-bis(2-nitrophenyl)pyrroles. Background technique Many 2-arylpyrrole and 2,5-diarylpyrrole compounds have important biological activities and are widely used as lead compounds in drug discovery and development. For example: the compound of formula I can be used as a selective ligand for α-adrenergic receptors (references: Pittala, V.; Romeo, G. et al. Bioorg. Med. Chem. Lett. 2006, 16, 150-153 ); the compound of formula II is a kind of β-secretase inhibitor, and is used as a lead compound in the drug development for the treatment of Alzheimer's disease (references: Cole, D.C.; Manas, E.S.et al.J.Med.Chem .2006,49,6158-6161); formula III compound is the lead compound of EP1 receptor antagonist (references: (a) Hall, A.; Atkinson, S.; Brown, S.H.et al.Bioorg.Med.Chem . Lett. 2006, 16, 3657-3662; (b) Hall, A.; Brown, S.H. et al. Bioorg. Med. Chem. Lett. 2007, 17, 732-735; (c) Hall...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D207/33C07D207/333C07D207/337
Inventor 胡跃飞朱锐王歆燕徐继民邵长伟
Owner 胡跃飞
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com