Patents
Literature
37 results about "Nucleophilic aromatic substitution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. The most important of these is the SNAr mechanism, where electron withdrawing groups activate the ring towards nucleophilic attack, for example if there are nitro functional groups positioned ortho or para to the halide leaving group.
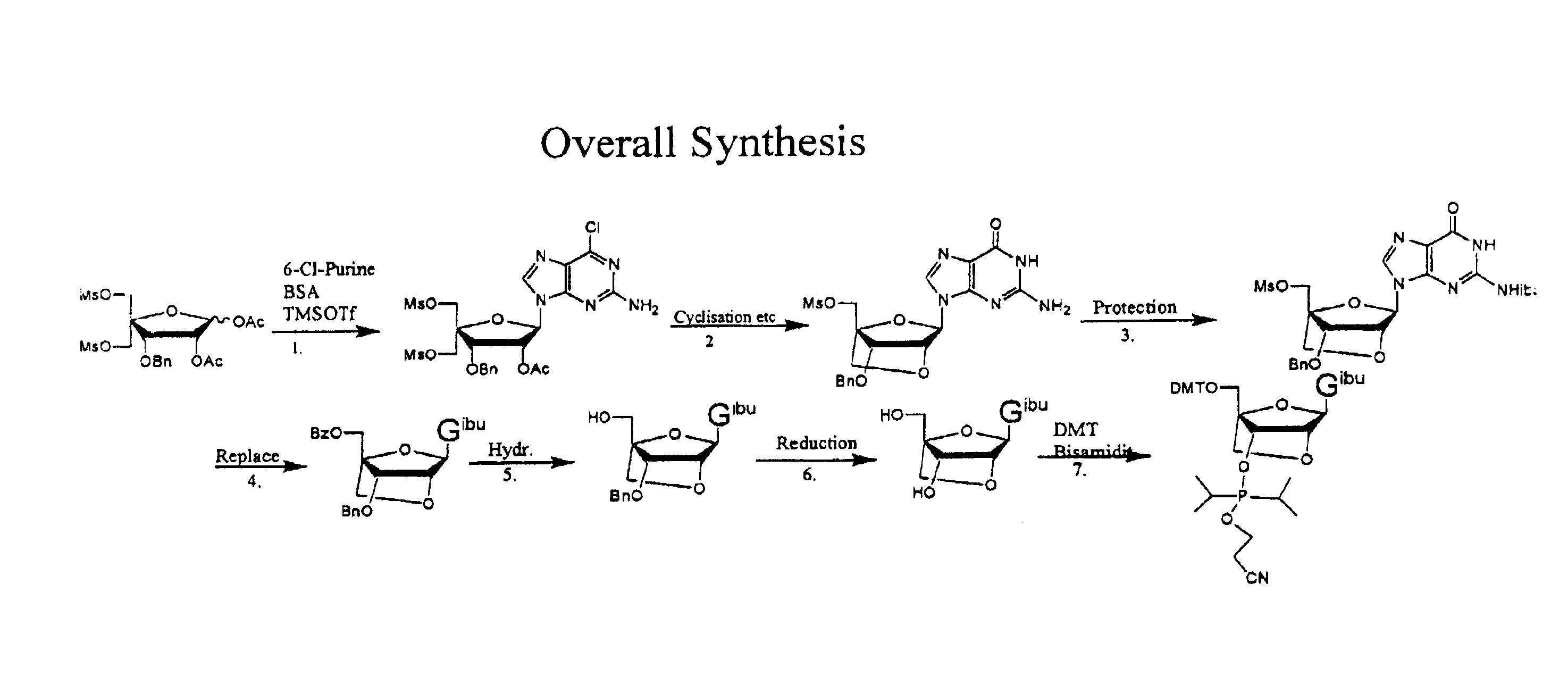
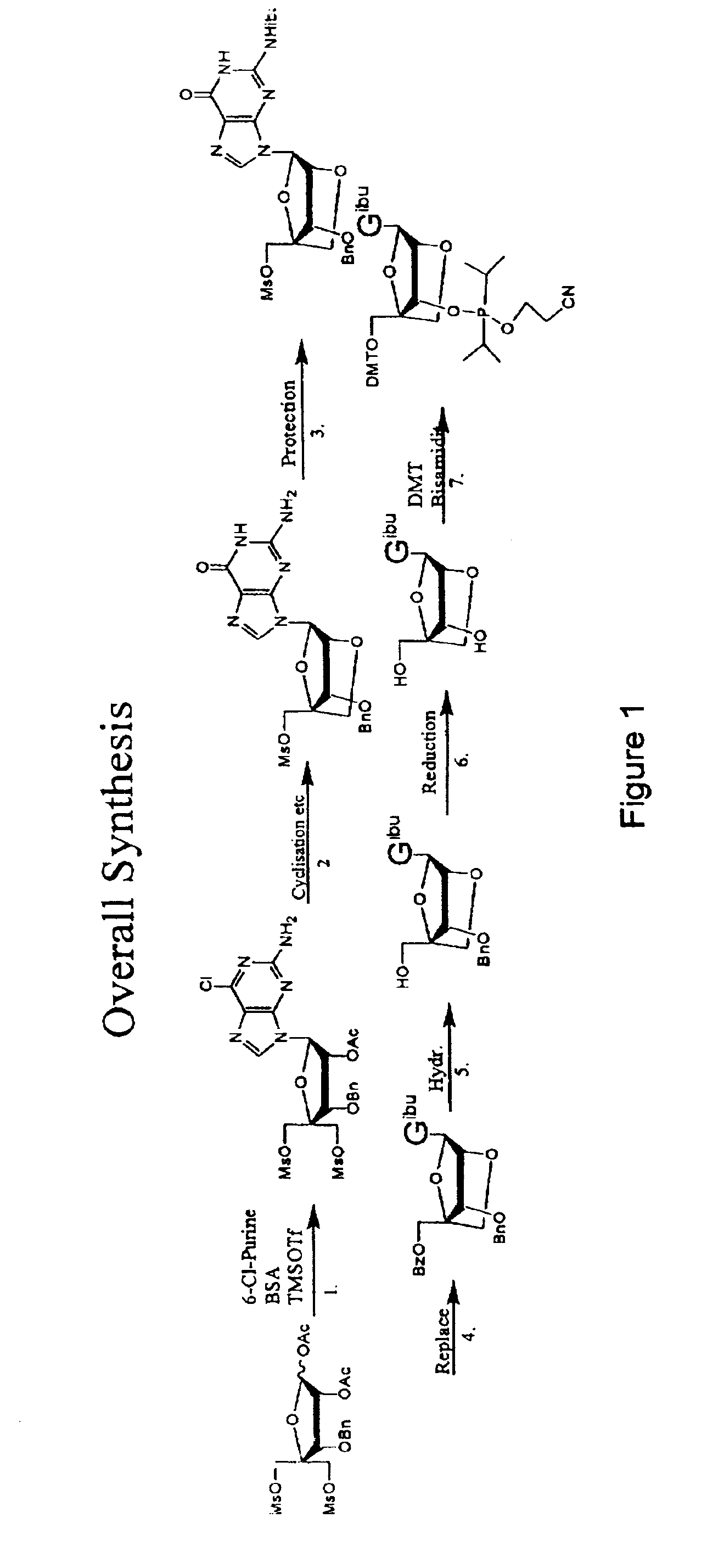
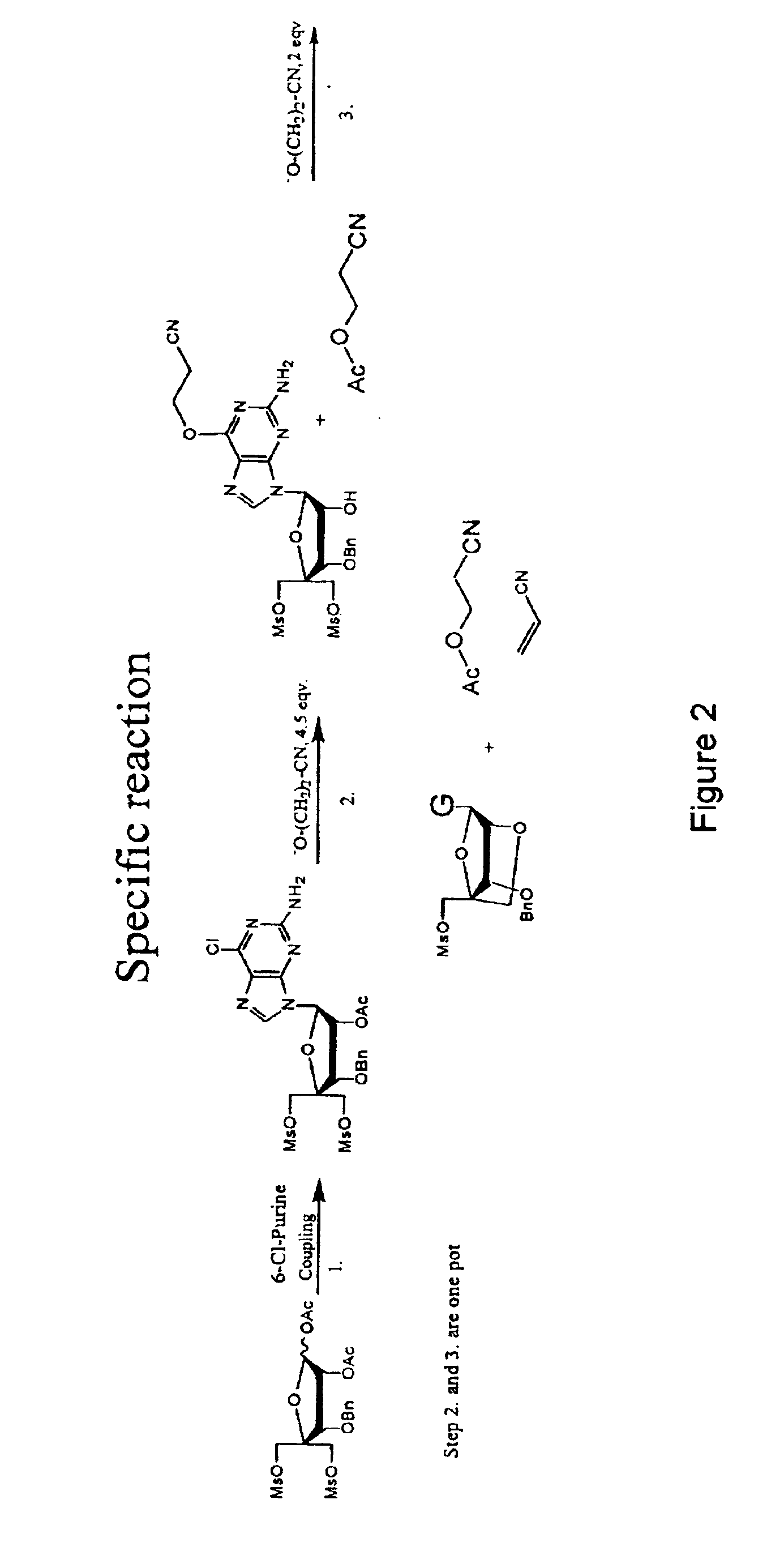
Synthesis of purine locked nucleic acid analogues
InactiveUS6998484B2Easy to convertEasy to liftSilicon organic compoundsSugar derivativesPurineLocked nucleic acid
The present invention relates to a new method for the synthesis of purine LNA (Locked Nucleic Acid) analogues which provides a higher overall yield. The method comprising a regioselective 9-N purine glycosylation reaction followed by a one-pot nucleophilic aromatic substitution reaction of the 6-substituent in the purine ring and simultaneous nucleophile-induced intramolecular ring closure of the C-branched carbohydrate to form novel purine LNA analogues. The novel strategy is illustrated by the synthesis of the novel compound (1S,3R,4R,7S)-7-benzyloxy-1-methanesulfonylmethyl-3-(guanin-9-yl)-2,5-dioxabicyclo[2.2.1]heptane which is easily converted into (1S,3R,4R,7S)-7-hydroxy-1-hydroxymethyl-3-((2-N-isobutyrylguanin-9-yl)-2,5-dioxabicyclo[2.2.1]heptane after isobutyryl protection of the 2-amino purine group and subsequent substitution of 1-methanesulfonyl with benzoate, debenzoylation and debenzylation.
Owner:SANTARIS PHARMA AS
Methods for synthesis of graphene derivatives and functional materials from asphaltenes
ActiveUS20160039678A1Low costFrom normal temperature solutionsOrganic chemistryElectrophilic aromatic substitutionGraphene derivatives
Embodiments described are directed to methods for the functionalization of asphaltene materials and to compositions made from functionalized asphaltenes. Disclosed is a method for synthesizing graphene derivatives, such as 2D single crystalline carbon allotropes of graphene and functional materials, such as sulfonic acid and its derivatives. Also disclosed is a method for the transformation of asphaltene into a source of graphene derivatives and functional materials, such as, 0D, 1D, 2D and combinations of 0D and 1D by utilizing chemical substitution reaction mechanism, such as, electrophilic aromatic substitution, nucleophilic aromatic substitution and Sandmeyer mechanism. Also disclosed are novel graphene materials comprising: acetylenic linkage and hydrogenated graphene. These novel materials, which may be produced by these methods, include, e.g.: 2D single crystalline carbon allotropes of graphene with asymmetric unit formulas C7H6N2O4, C6H4N2O4, C7H7O3S− H3O+, C7H7O3SH+, and a 2D single crystal with asymmetric unit formula (Na6O16S4)n.
Owner:TANIMOLA OLANREWAJU W
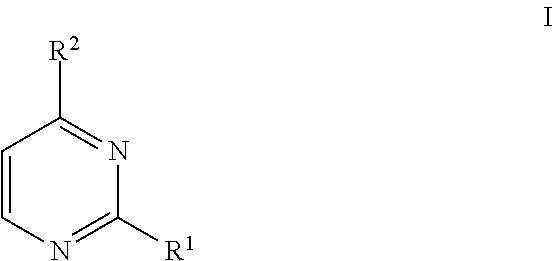
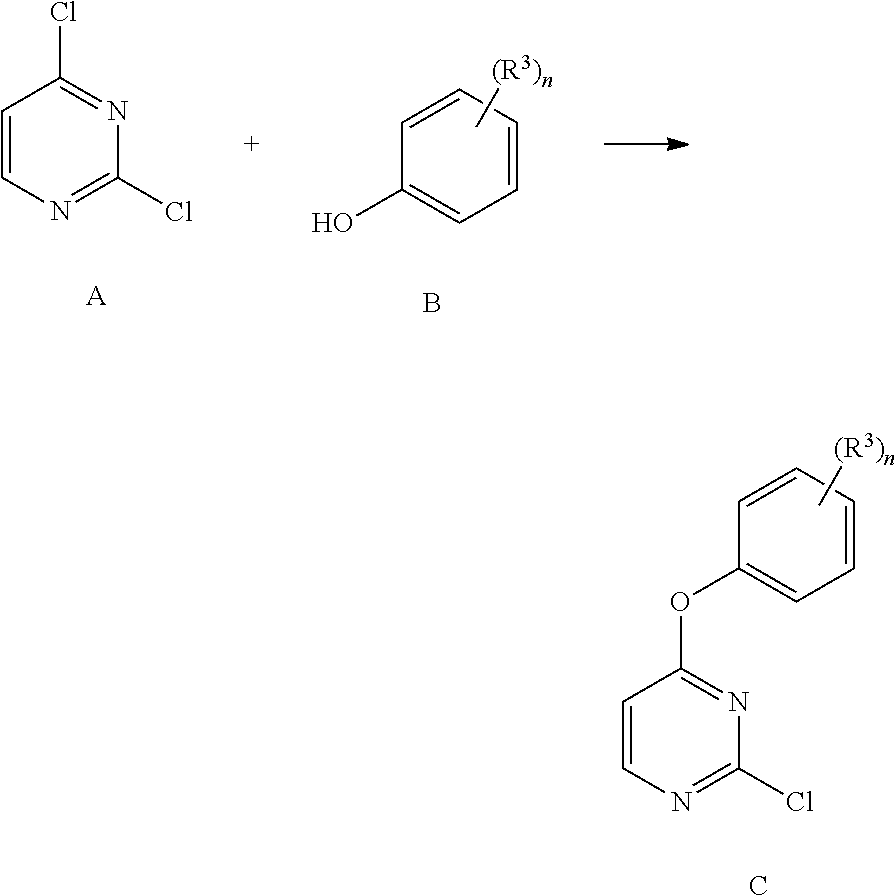
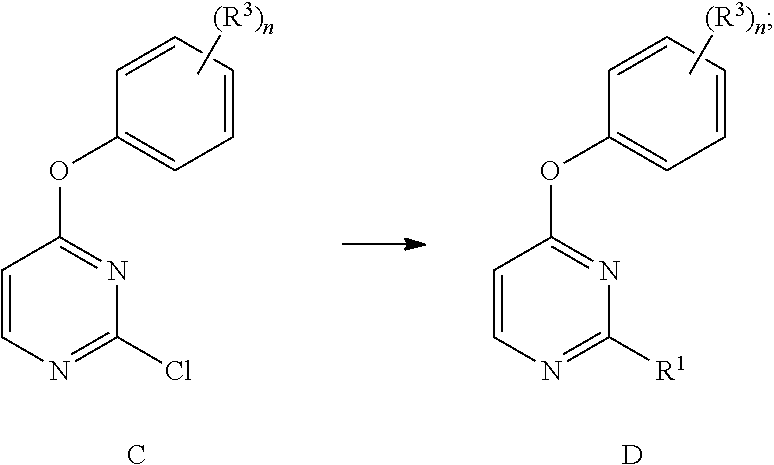
Methods of regioselective synthesis of 2,4-disubstituted pyrimidines
Owner:VERTEX PHARMA INC
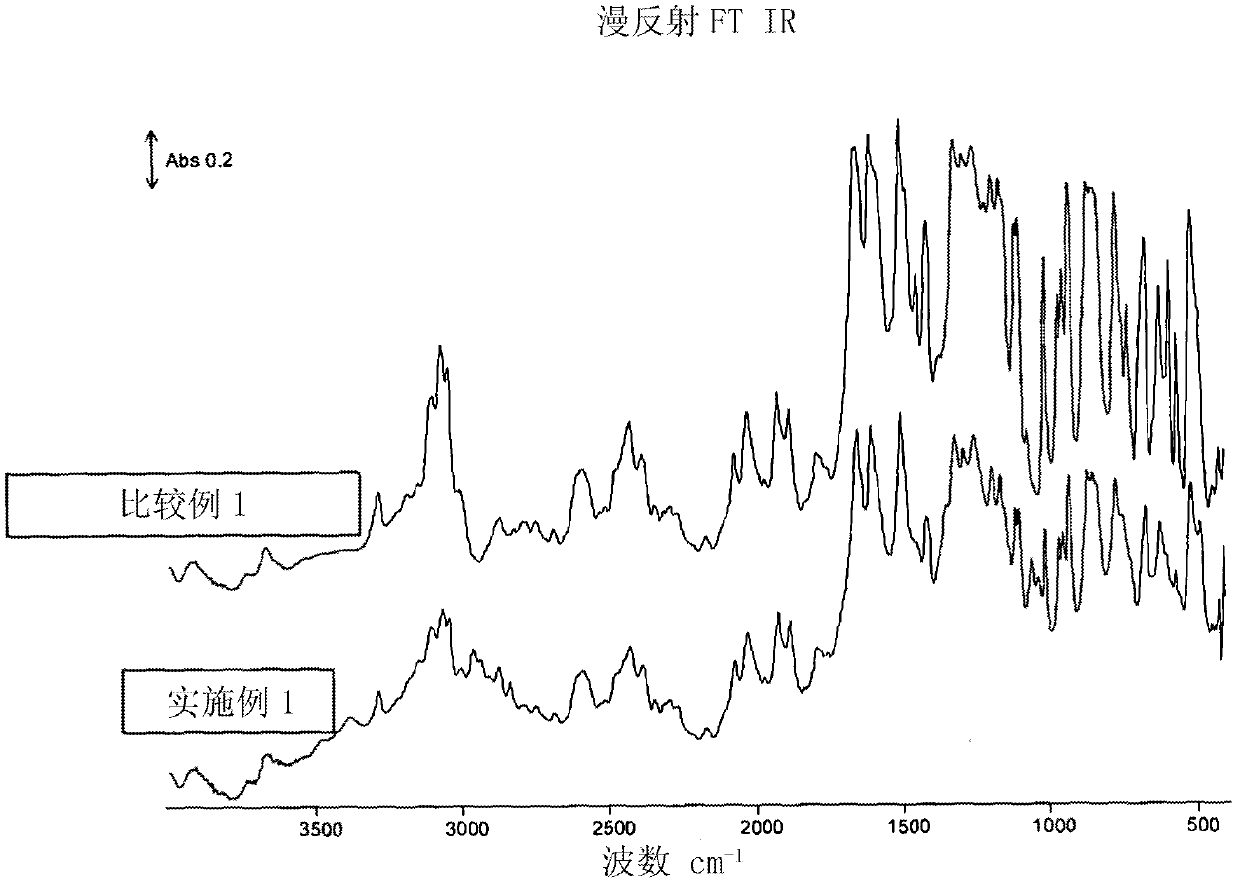
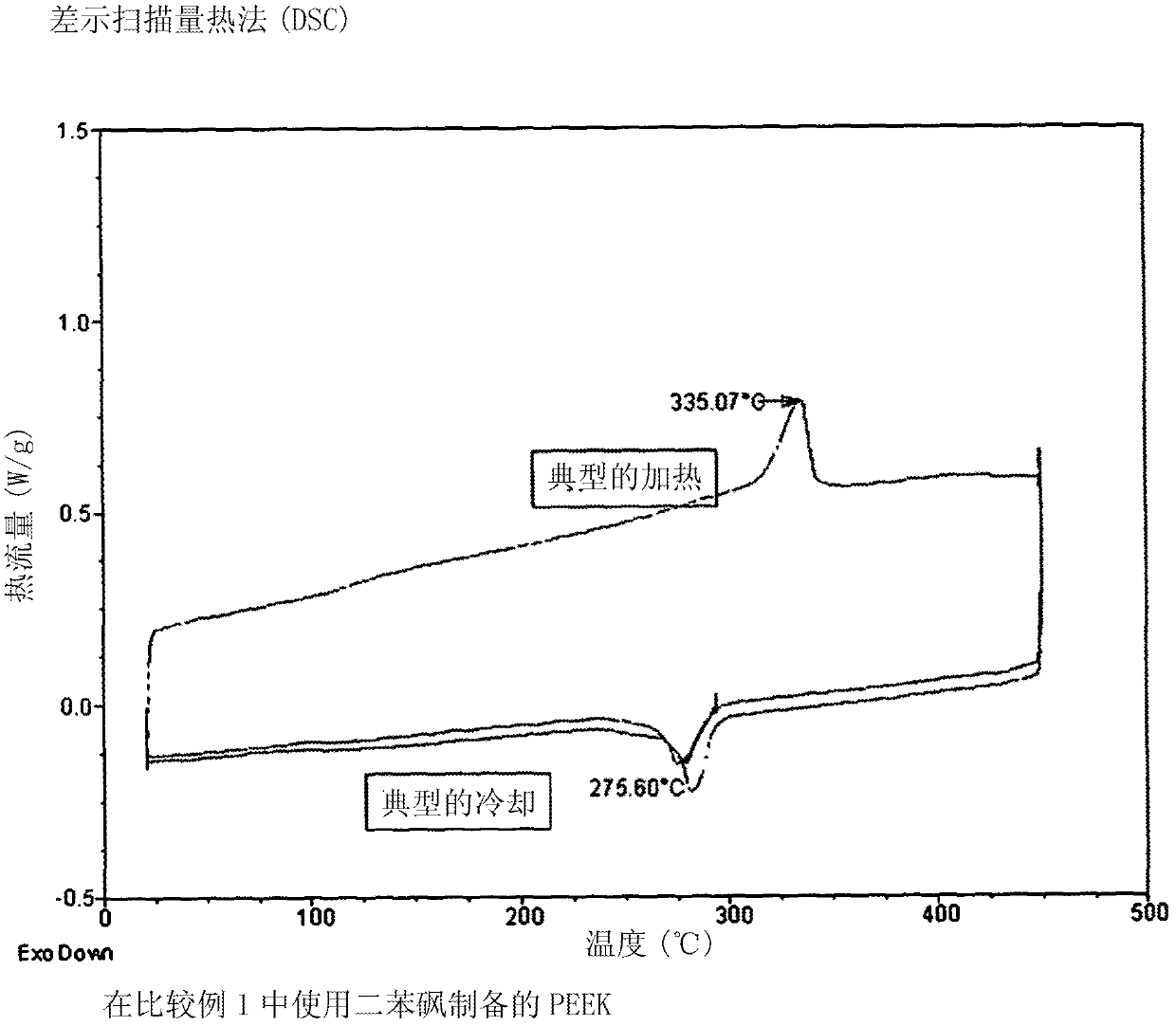
Sulfonated polyether ether ketone ketone, film utilizing the same, and method for manufacturing the same
A series of crosslinkable sulfonated poly(ether ketone)s containing cycloalkenyl groups were synthesized by aromatic nucleophilic substitution reaction. To decrease the swelling of fuel cell membranes, crosslinking of theses polymers by radical polymerization has been explored. These polymeric films exhibit good thermal and oxidative stability, and good dimensional stability in hot water. The proton conductivity of one example at room temperature is 7.52*10−2 S / cm. The results showed that these materials containing cycloalkenyl groups are possible inexpensive candidate materials for proton exchange membranes in fuel cell applications.
Owner:IND TECH RES INST +1
Methods of regioselective synthesis of 2,4-disubstituted pyrimidines
Owner:VERTEX PHARMA INC
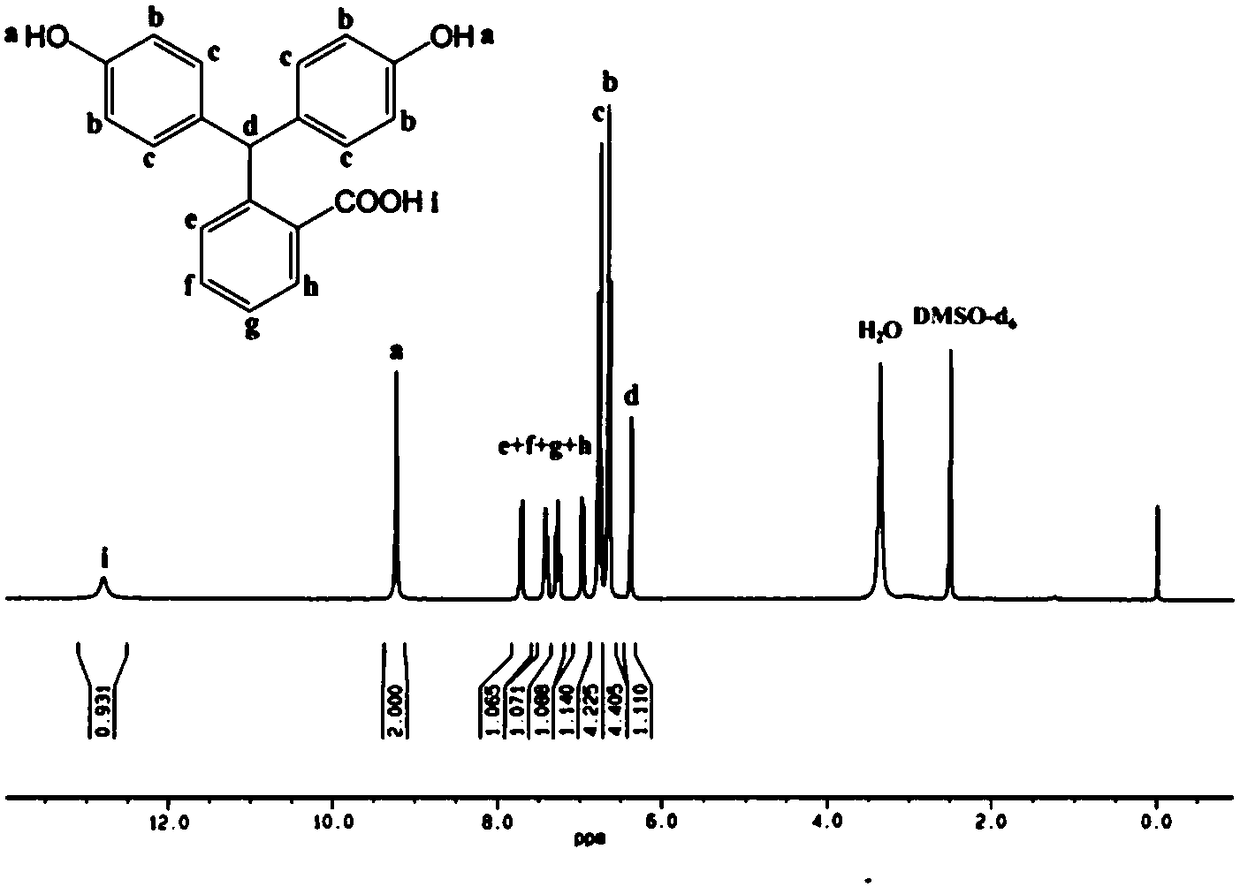
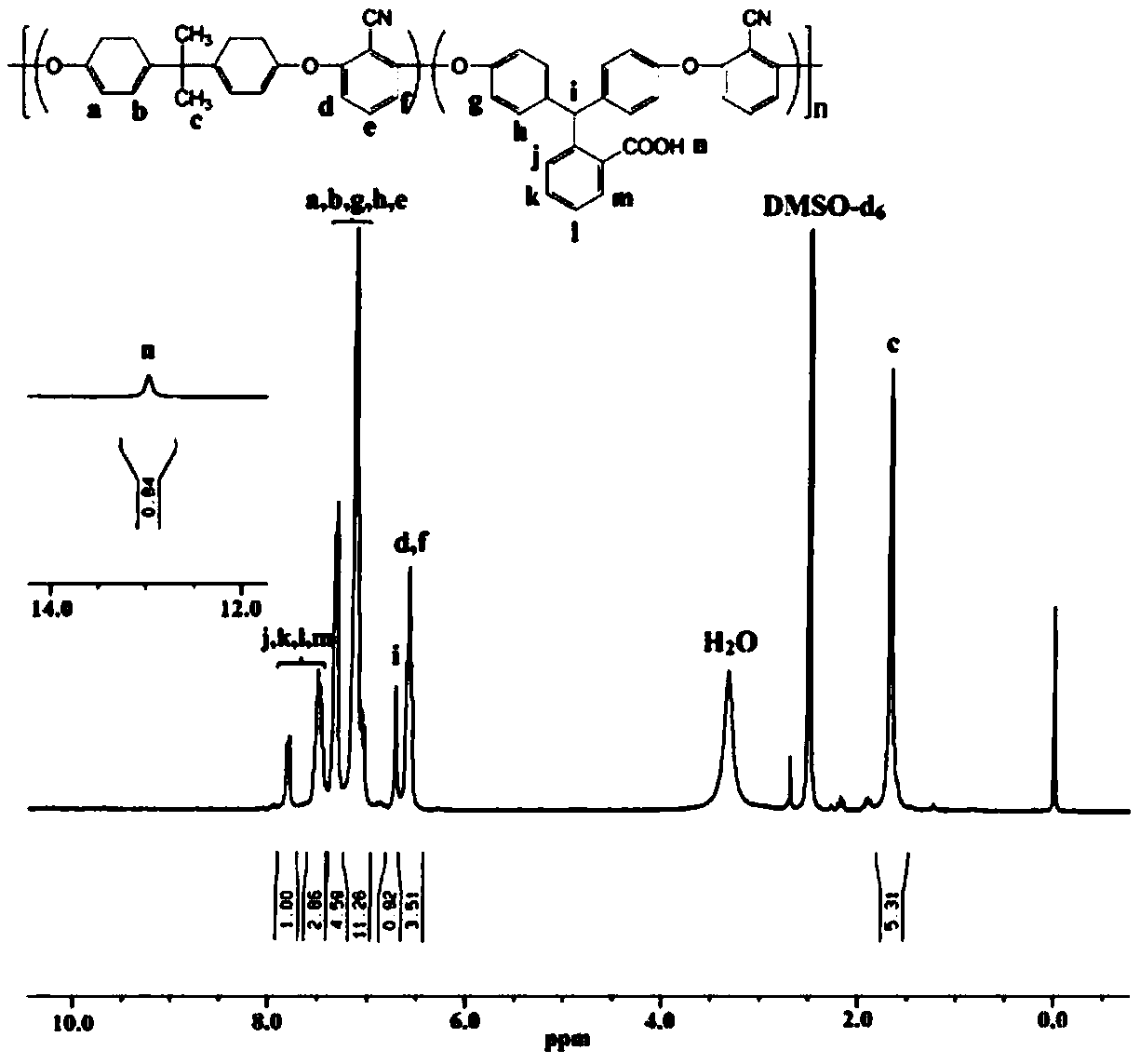
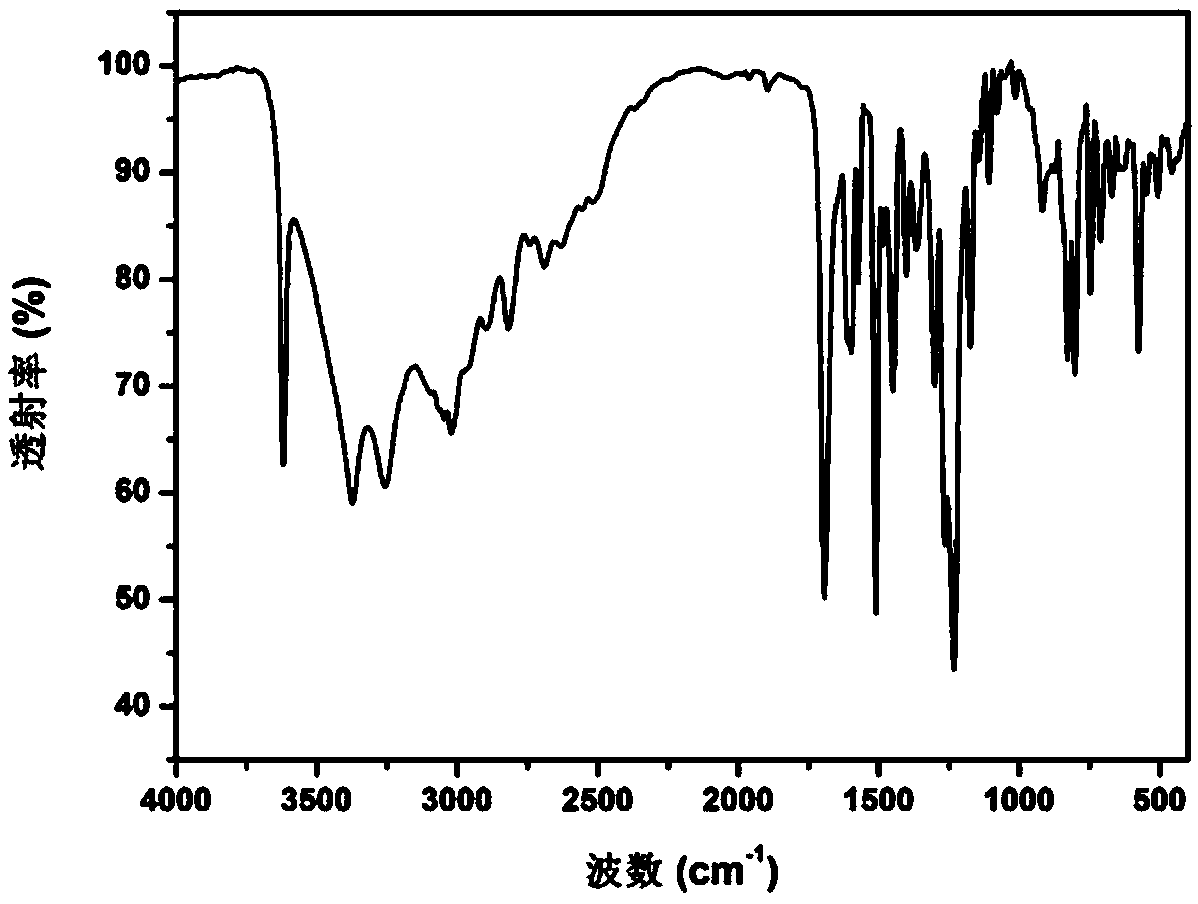
Preparation method of high-performance carboxyl functionalized poly (arylene ether nitrile)
InactiveCN109503826AWith functional diversityHigh glass transition temperatureSolubilityPolymer science
The invention discloses a preparation method of high-performance carboxyl functionalized poly (arylene ether nitrile). The method comprises the following steps: synthesizing a carboxyl-containing monomer phenolphthalein by taking phenolphthalein and a zinc powder as raw materials, performing stepwise polymerization by virtue of nucleophilic aromatic substitution, and introducing the phenolphthalein structural units into a main chain of the poly (arylene ether nitrile), so as to obtain the high-performance carboxyl functionalized poly (arylene ether nitrile) copolymer. The polymer has a high glass-transition temperature, excellent heat stability and thermo-oxidative stability, high mechanical strength and good solubility in a polar solvent, has strong absorption in an ultraviolet region of280-330nm and also has blue-fluorescence functional characteristics under ultraviolet excitation, so that the poly (arylene ether nitrile) has functional diversity, and the application range of the product is widened. The theoretical direction and technical support are provided for simple and high-efficiency industrial production of the high-performance poly (arylene ether nitrile), the gap of functional development of the poly (arylene ether nitrile) is filled for preparing high-performance reactive functional composite materials, and the preparation method has excellent application prospects.
Owner:CHONGQING UNIV OF TECH
Dispersant for use in synthesis of polyaryletherketones
A method for forming a polyaryletherketone is described. More particularly, a reaction mixture is initially supplied to the reactor vessel that contains one or more precursor monomers. A heteroaryl compound is also added to the reaction mixture. The reaction can be carried out according to, e.g., an electrophilic aromatic substitution reaction or a nucleophilic aromatic substitution reaction. The heteroaryl compound can serve as a dispersant to the polymer as it is formed. This minimizes the likelihood of gelling of the product polymer within the reactor vessel and limits the impact of process disruptions on the production of the polyaryletherketone.
Owner:TICONA LLC
Process for preparing a compound
InactiveUS20090082597A1Reduce the amount requiredHigh resolutionOrganic compound preparationAmino-hyroxy compound preparationAlcoholNucleophilic aromatic substitution
The invention relates to the use of copper-catalyzed nucleophilic aromatic substitution reaction for preparing 3-aryloxy-3-arylpropylamines and more specifically to a method of preparing certain 3-aryloxy-3-arylpropylamines and the pharmaceutically acceptable addition salts thereof, comprising reacting an amino alcohol with a haloaromatic compound in the presence of a base and a catalytic copper source, and in the absence of a separate ligand.
Owner:FERMION
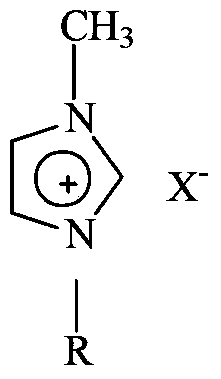
Synthesis method using ionic liquids
InactiveCN102471477AImprove solubilityHigh purityOrganic-compounds/hydrides/coordination-complexes catalystsIonNucleophilic aromatic substitution
The disclosures herein provide a process for conducting a nucleophilic aromatic substitution reaction in an ionic liquid and forming a polymeric material.
Owner:INVISTA TECHNOLOG IES S A R L
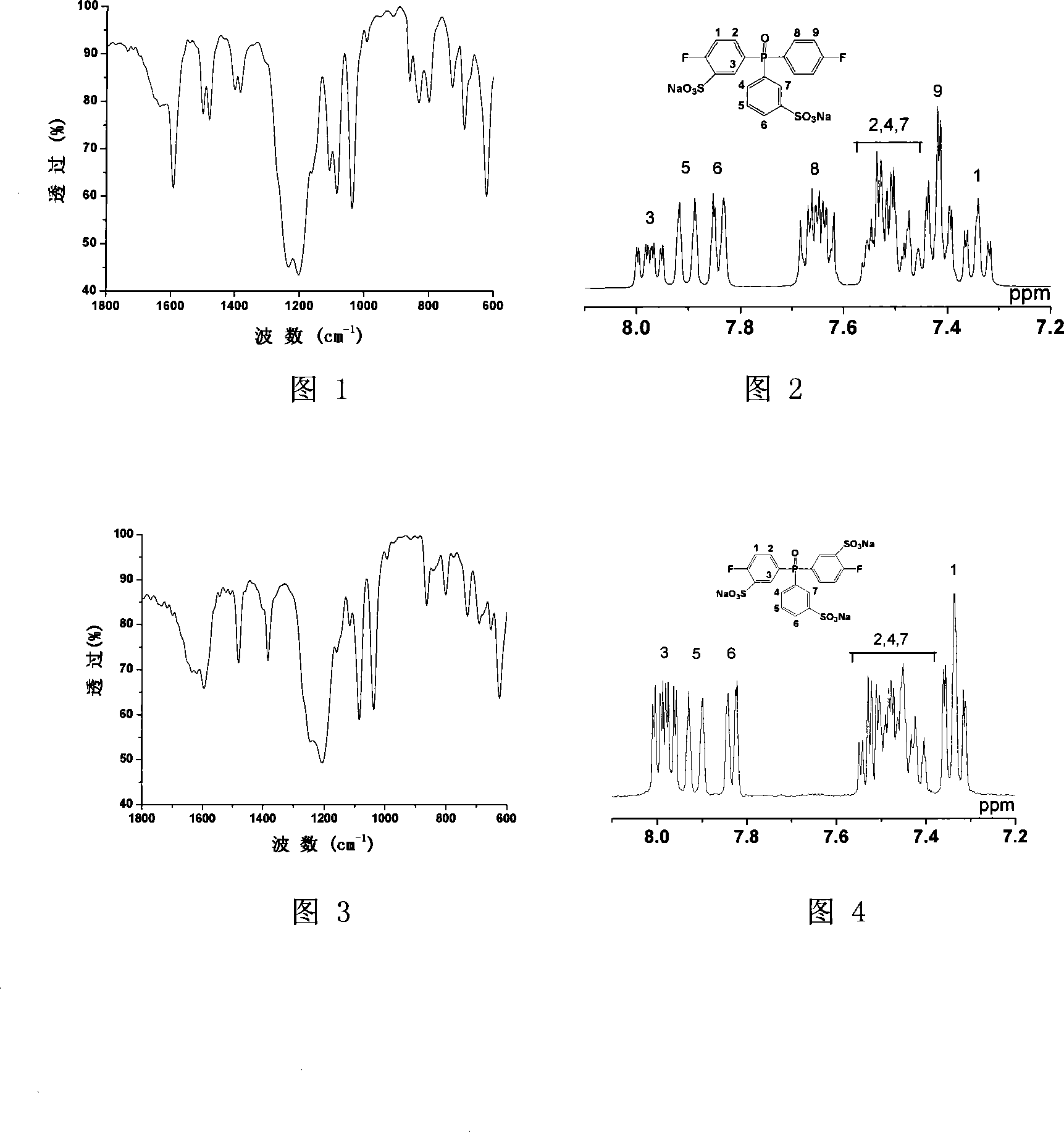
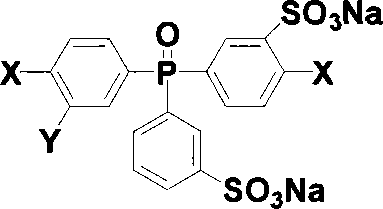
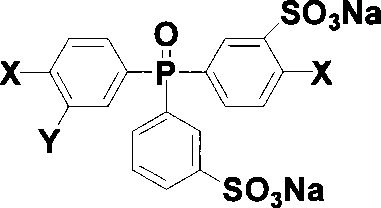
Sulfonated compound containing phosphinyl structure and preparation method thereof
InactiveCN101168548AEnhanced interactionImprove performanceGroup 5/15 element organic compoundsHydrogenHalogen
The invention discloses sulphonated compound including a phosphine oxide structure, and the preparation method thereof, in particular to 3-sodium sulfonate-4-halogen phenyl-3a-sodium sulfonate phenyl-4a-halogen phenyl phosphine oxide and di (3-sodium sulfonate-4-halogen phenyl)-3a-sodium sulfonate phenyl phosphine oxide and the preparation method thereof. The compound is obtained by taking di (4-halogen phenyl) phenyl phosphine oxide as the raw material through the sulphonation reaction, and the structural formula is shown as follows: the compound can have the nucleophilic aromatic substitution reaction with a bifunctional monomer including two active hydrogens under the alkaline condition, thus sulphonated polymer including a phosphine oxide structural unit is obtained, the performance of the sulphonated polymer is excellent, and the sulphonated polymer yearns for being used for proton exchange membrane materials or other functional materials. X is F, Cl, Br, or I, and Y is H or -SO3Na.
Owner:SHANGHAI JIAO TONG UNIV
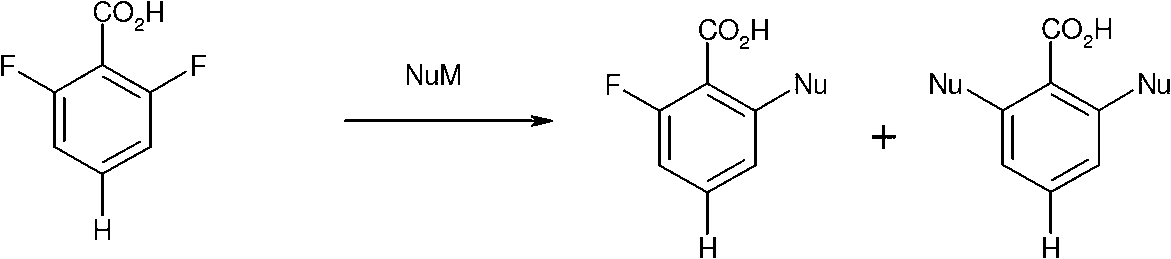
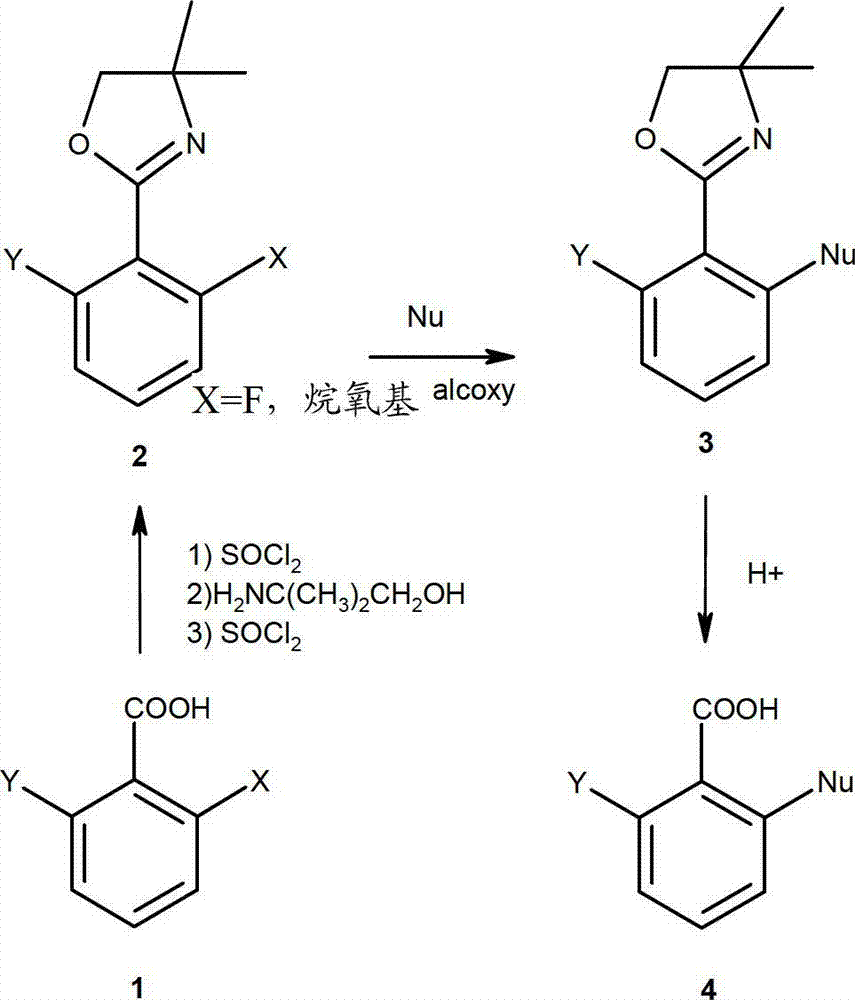
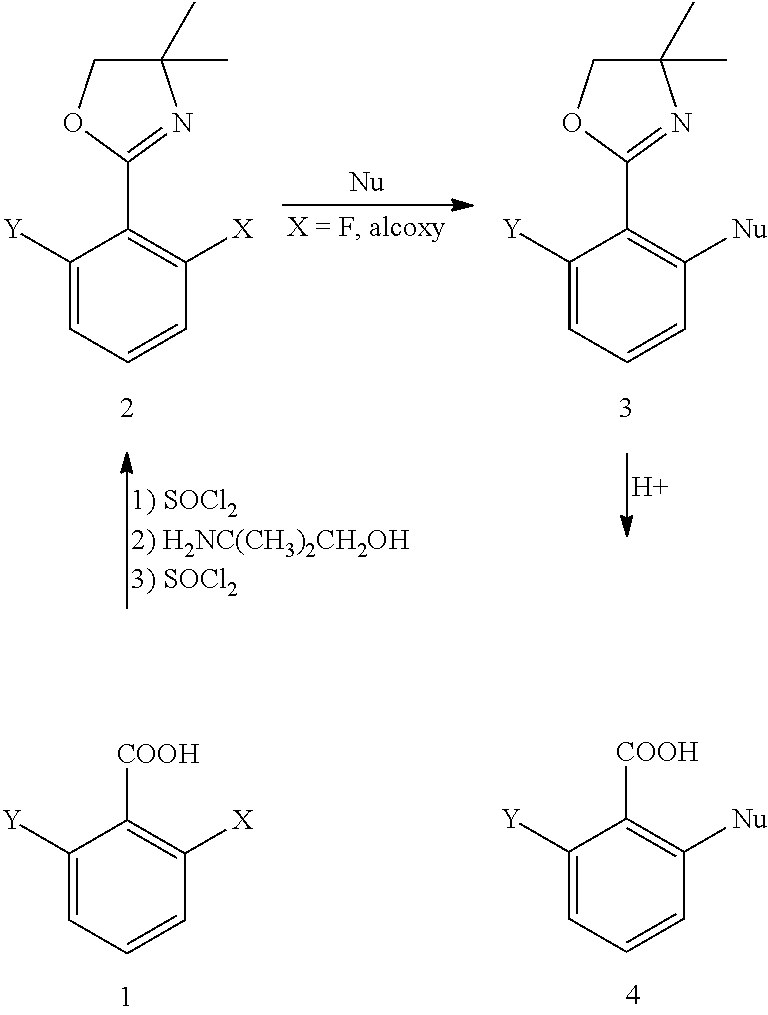
Method for preparing chemical compounds of interest by nucleophilic aromatic substitution of aromatic carboxylic acid derivatives supporting at least one electro-attractive group
InactiveCN102985399AOrganic compound preparationAmino-carboxyl compound preparationLeaving groupCarboxyl radical
The present invention relates to a method for preparing aromatic carboxylic acid derivatives by nucleophilic aromatic substitution, which involves reacting an aromatic carboxylic acid derivative supporting only one carboxyl function, or one of the salts thereof, said carboxylic acid derivative supporting, orthogonally to the carboxyl function, a splitting group which is an atom of fluorine or chlorine or an alcoxy group, chiral or otherwise and, in the latter case, a methoxy group is preferred; said carboxylic acid derivative being substituted by at least one electro-attractive group other than the splitting group, preferably by a fluorine atom, with a MNu reagent, wherein M is a metal and Nu is an optionally chiral nucleophile, said nucleophilic aromatic substitution reaction being carried out without a catalyst and without a step of protecting / unprotecting the acid function of the initial compound, said method being selective in that the reaction leads to the formation of ketone derivatives in a very minority fashion during the reaction.
Owner:曼恩大学
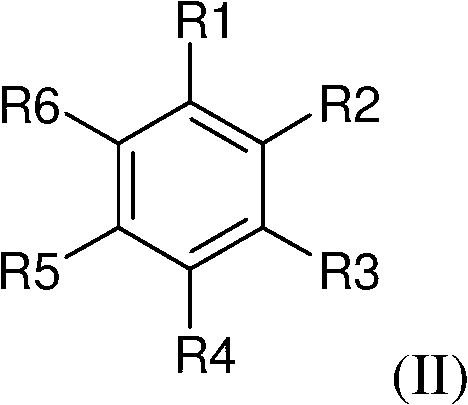
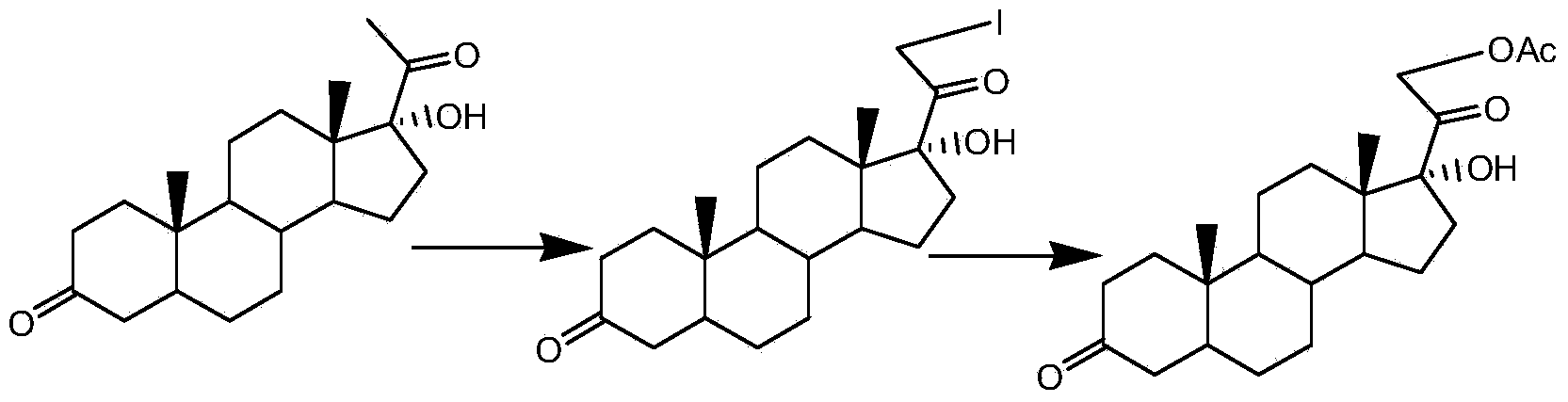
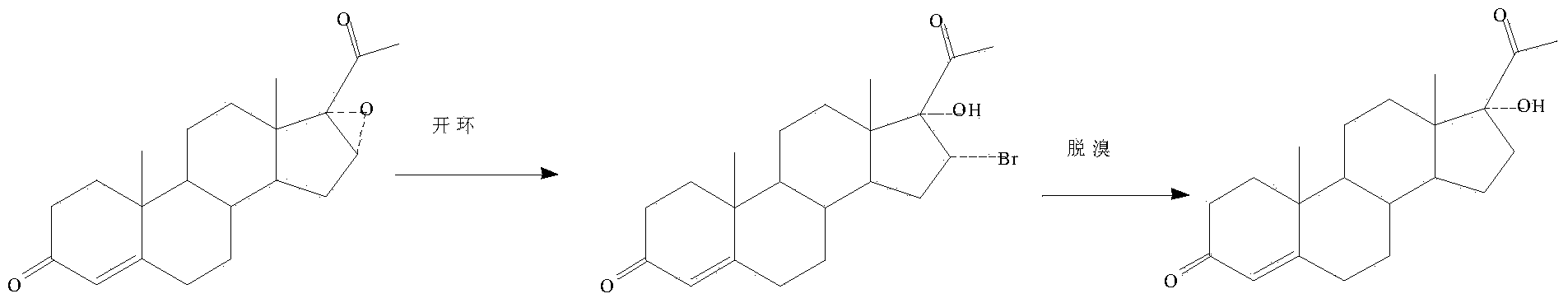
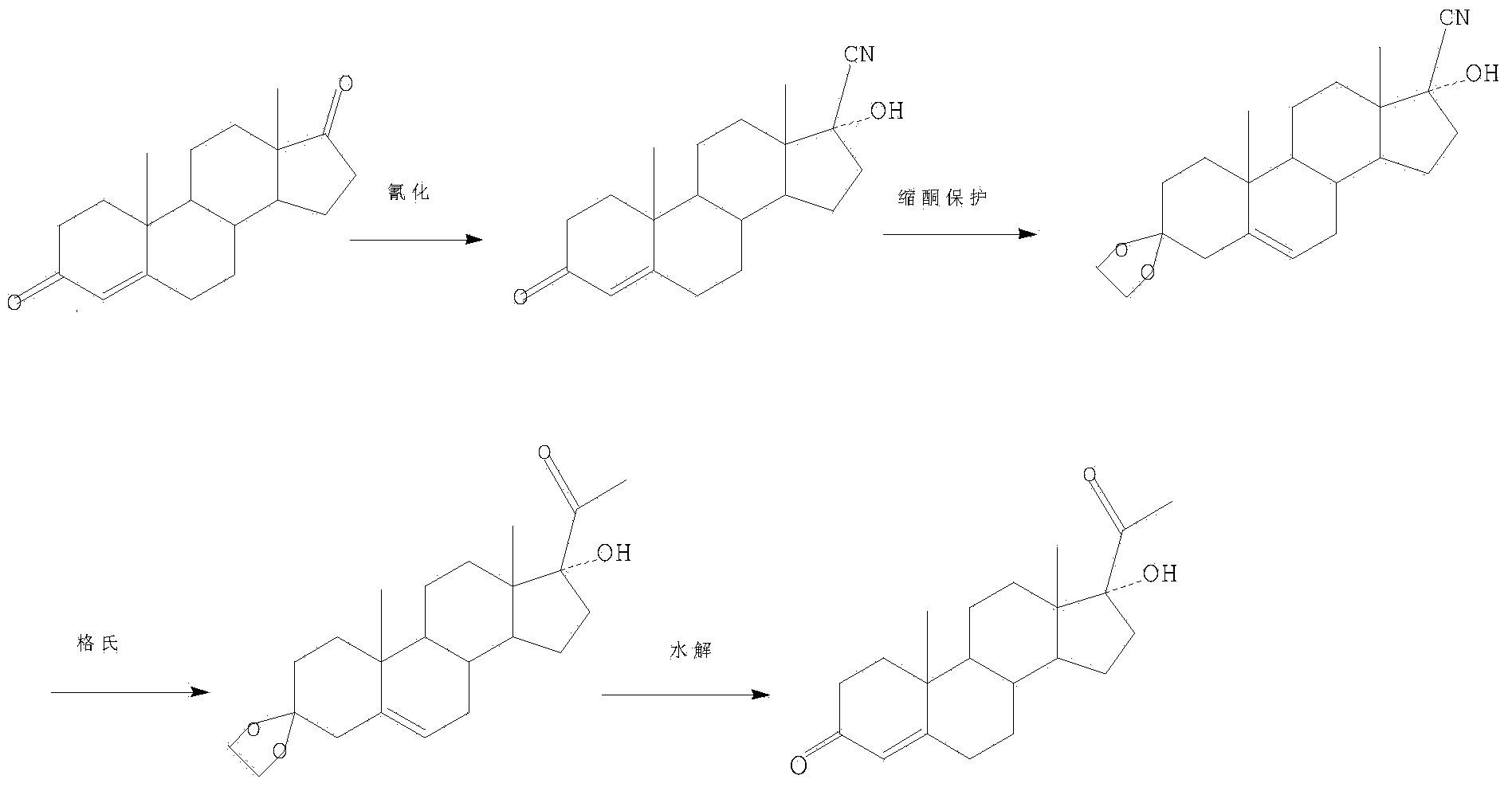
Preparation method of 17hydroxy-pregnane-4-alkene-3,20-diketone-21-acetic ester
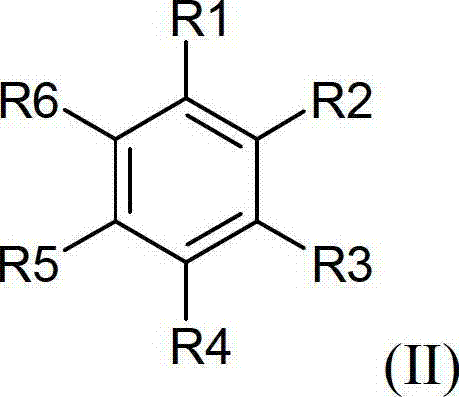
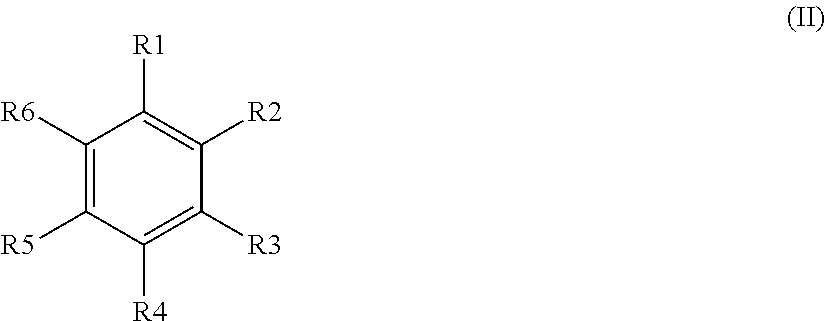
The invention provides a preparation method of a 17hydroxy-pregnane-4-alkene-3, 20-diketone-21-acetic ester. The preparation method is characterized in that a compound as shown in a formula (II) described in the specification is subjected to cyanation, protection, nucleophilic substitution and esterification reaction, and the 17hydroxy-pregnane-4-alkene-3, 20-diketone-21-acetic ester as shown in the formula (I) is obtained. The preparation method provided by the invention has the advantages that the process is simple, the cost is low, the yield is high and stable, and the preparation method is suitable for industrial production.
Owner:ZHEJIANG XIANJU JUNYE PHARM CO LTD
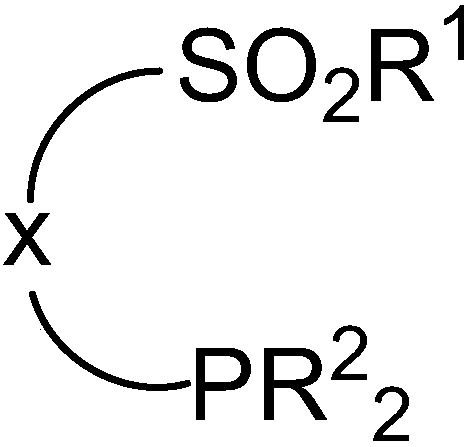
Organic phosphine compound with sulfonyl functional group, as well as preparation method and application thereof
InactiveCN108484668AQuick buildAccelerated discoveryGroup 5/15 element organic compoundsSulfonyl chlorideLeaving group
The invention discloses an organic phosphine compound with a sulfonyl functional group, as well as a preparation method and application thereof. The organic phosphine compound with the sulfonyl functional group comprises a sulfonyl group, a phosphine-containing group and the functional group capable of leaving through nucleophilic substitution. During preparation, sulfonyl chloride and a reagent with a leaving group are reacted, and the functional group capable of leaving through nucleophilic substitution is introduced; then a carbon phosphine bond is built through nucleophilic substitution reaction or coupling reaction, and the phosphine-containing group is introduced. The organic phosphine compound with the sulfonyl functional group provided by the invention can be subjected to nucleophilic reaction with a nucleophilic reagent so as to quickly build a ligand library, provides a favorably technology for designing and synthesizing ligands, is good for accelerating the discovery and theoptimization of the reaction, and can be applied to nucleophilic substitution reaction with the nucleophilic reagent so as to build the phosphine-containing compound.
Owner:SUN YAT SEN UNIV
Cyclotriphosphazene derivative containing sulfydryl group and preparation method thereof
ActiveCN103570764AMild reaction conditionsEasy to controlGroup 5/15 element organic compoundsProcedure AgentsReaction temperature
The invention relates to a cyclotriphosphazene derivative containing a sulfydryl group and a preparation method thereof, and belongs to the technical field of processing aids. The method comprises the following steps: adding thiophenol and a solvent to a reactor in an inert gas atmosphere; simultaneously, slowly adding a sodium compound to stir, and adding phosphonitrilic chloride trimer, so as to obtain reaction liquid, wherein the reaction temperature is 20-120 DEG C; leaching a reaction by-product, and removing the solvent by evaporating; and finally obtaining the cyclotriphosphazene derivative containing the sulfydryl group. By adopting the preparation method, the nucleophilic substitution is carried out by using sulfydryl and the phosphonitrilic chloride trimer; a hexasubstituted cyclotriphosphazene derivative (named) containing the sulfydryl group, which is accompanied with a little of incompletely-substituted penta-substitution product, is successfully synthetized; the molar ratio of the hexasubstituted cyclotriphosphazene derivative to the incompletely-substituted penta-substitution product is 2.61:1. The preparation method disclosed by the invention is mild in reaction condition, and has the characteristics of being easy to control, good in repeatability, high in yield and the like.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
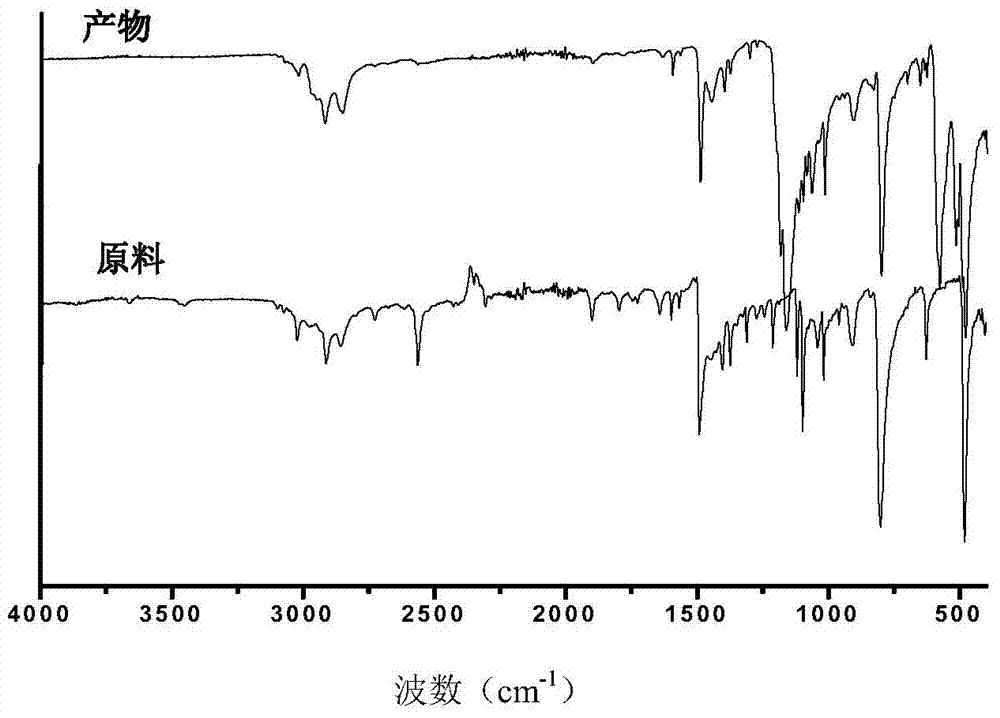
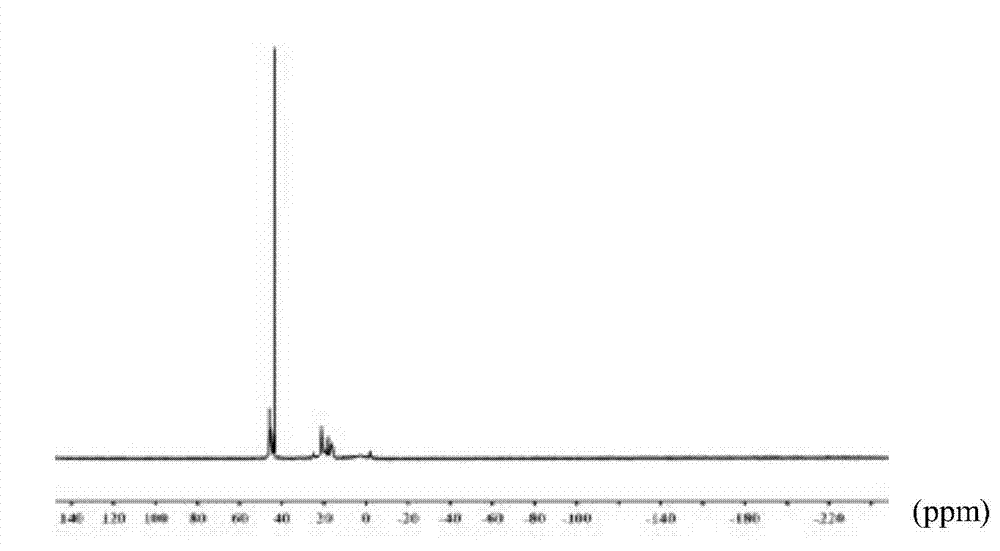
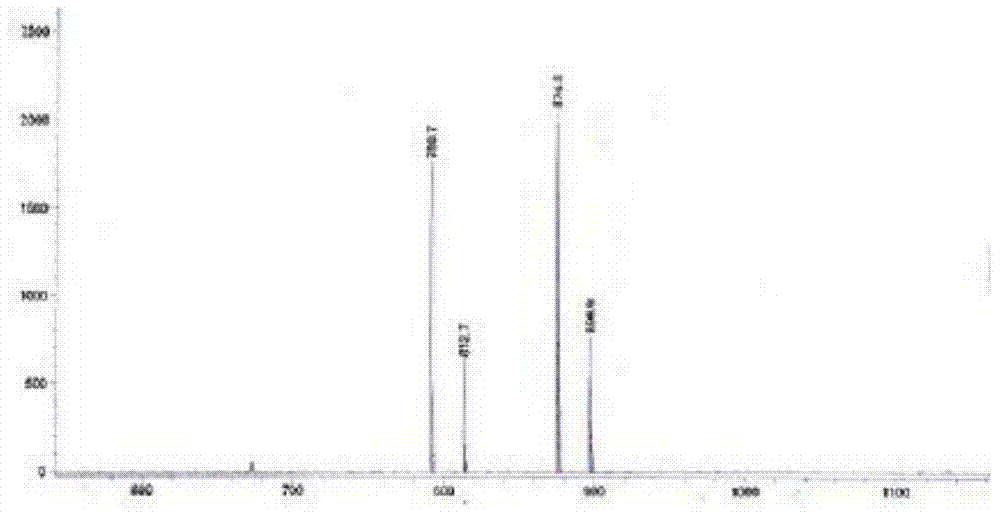
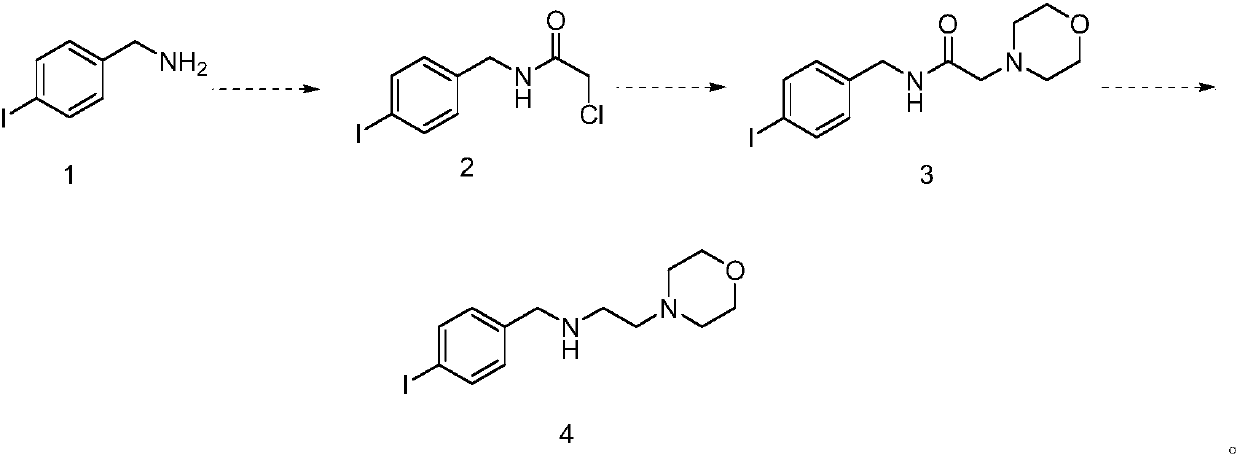
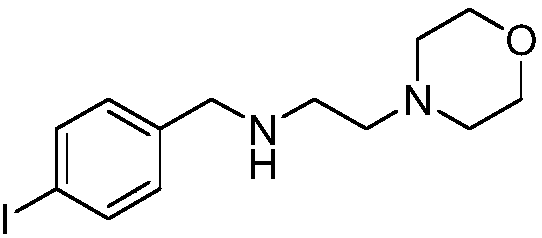
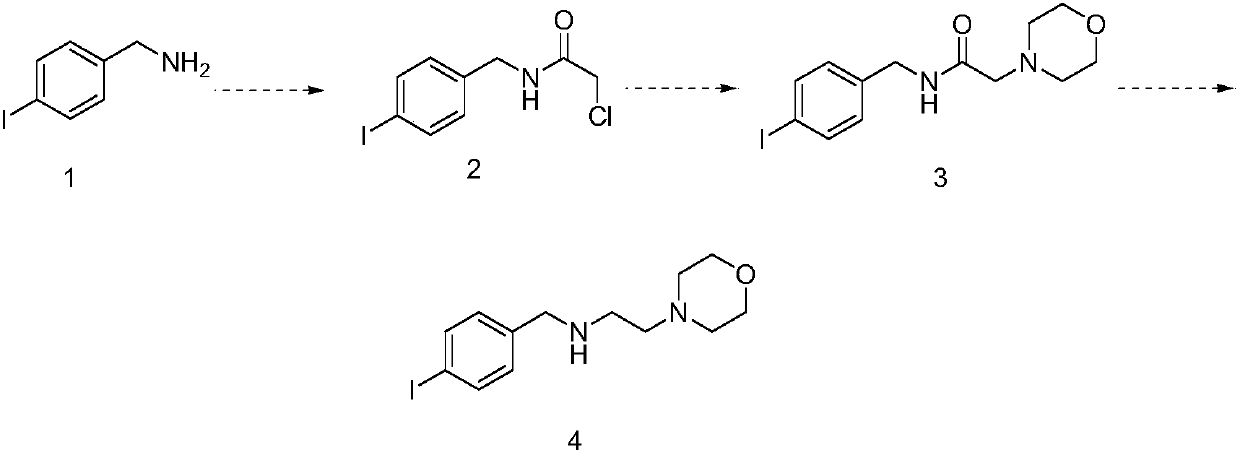
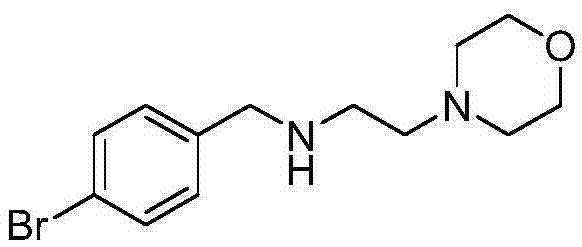
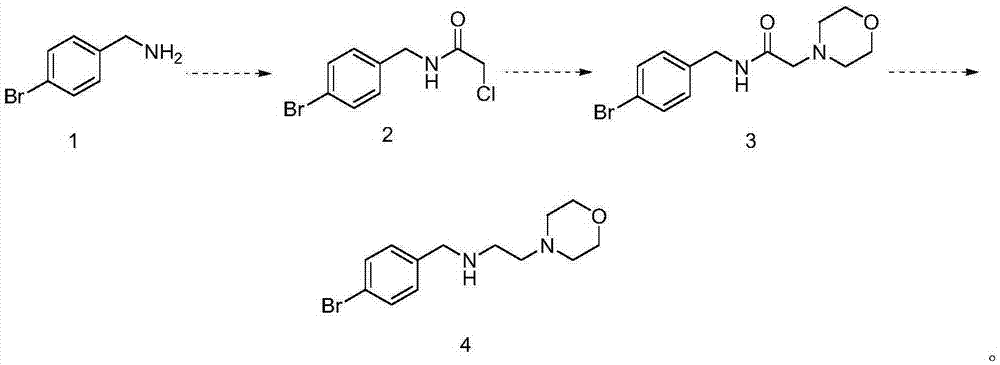
Preparation method of morpholine compound
The invention belongs to the field of organic synthesis, and in particular relates to a preparation method of a morpholine compound, i.e., N-(4-iodobenzyl)-2-morpholino ethylamine. According to the preparation method, 4-iodobenzylamine is used as a raw material, and the target product is obtained by carrying out acylation, nucleophilic substitution and reduction on the raw material. The compound can enrich organic synthesis molecular blocks.
Owner:湖南华腾制药有限公司
Method for preparing chemical compounds of interest by aromatic nucleophilic substitution
InactiveCN102958892AAvoid purification/removalOrganic compound preparationAmino-carboxyl compound preparationLeaving groupOrtho position
The aim of the invention is to provide a method for preparing carboxylic acid derivatives by aromatic nucleophilic substitution, in which a carboxylic acid derivative having a single carboxyl functional group, or one of the salts thereof, said carboxylic acid derivative having, in the ortho position of the carboxyl functional group, a leaving group, which is preferably an atom of fluorine or of chlorine or an alkoxy group, chiral or not, preferably a methoxy group, said carboxylic acid derivative not being substituted by an electroattractive group other than the leaving group if any; is reacted with a reactant MNu, where M is a metal and Nu is a nucleophile, chiral or not, said aromatic nucleophilic substitution reaction being carried out without a catalyst and without a step of protecting / deprotecting the acid functional group of the starting compound.
Owner:曼恩大学
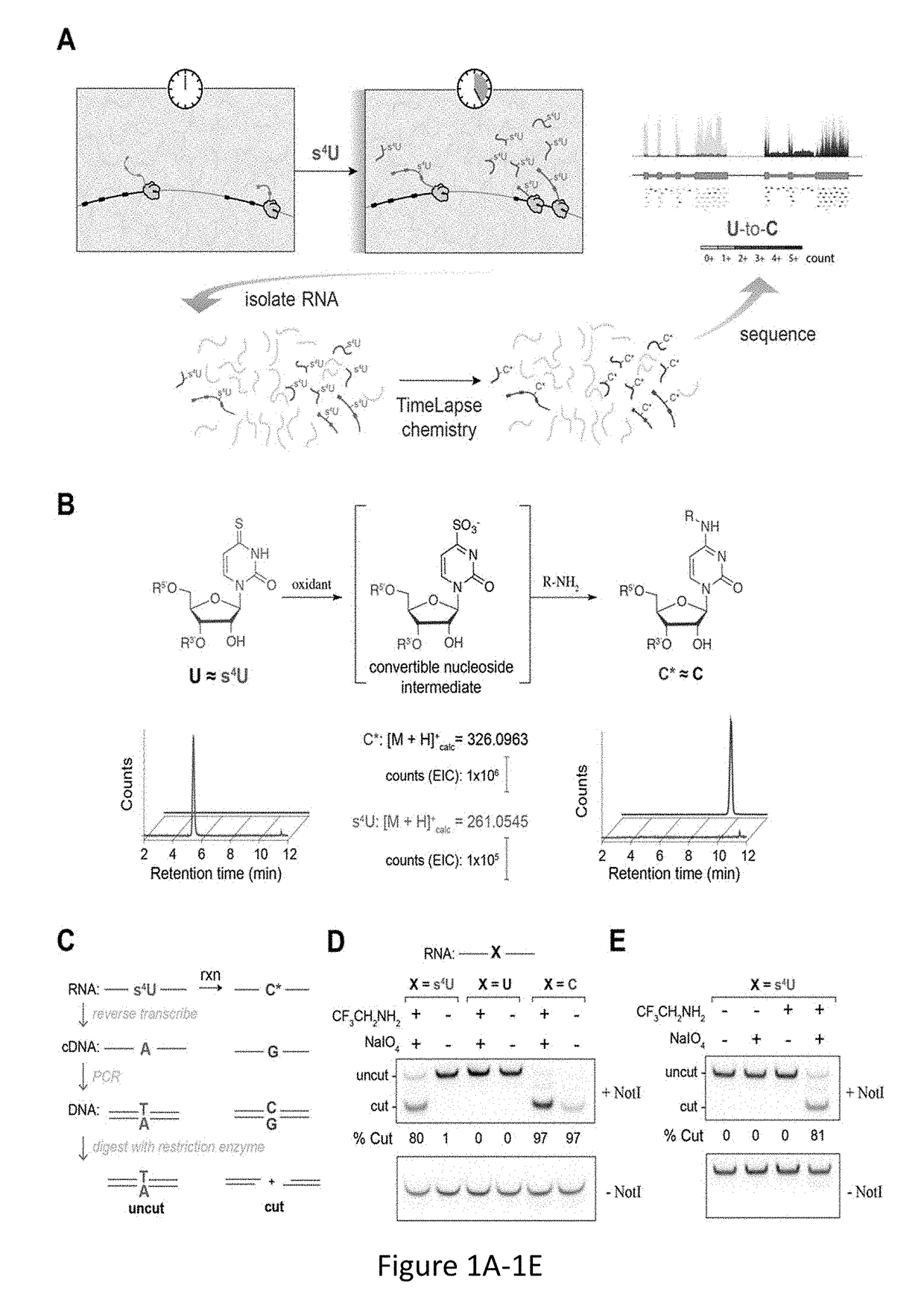
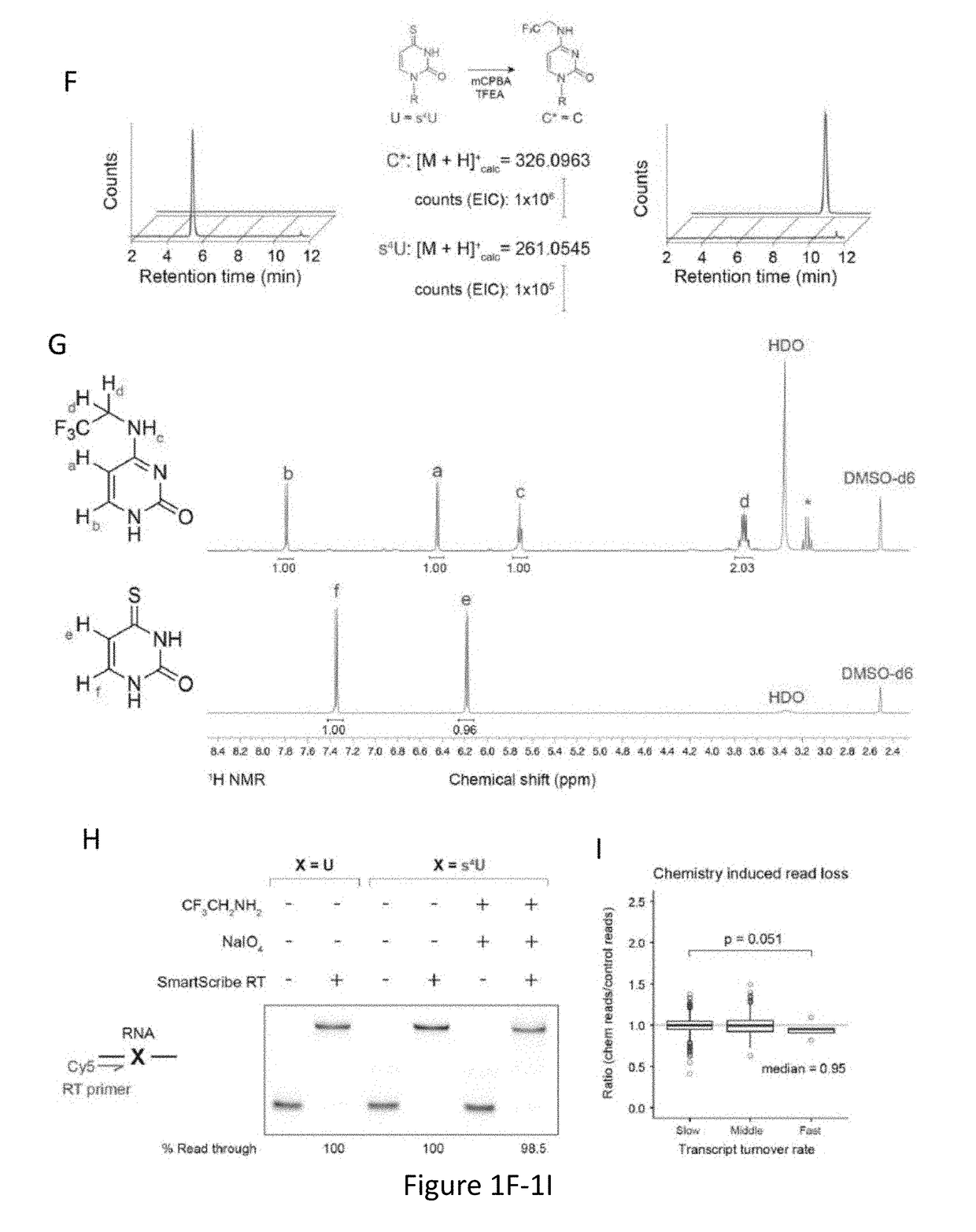
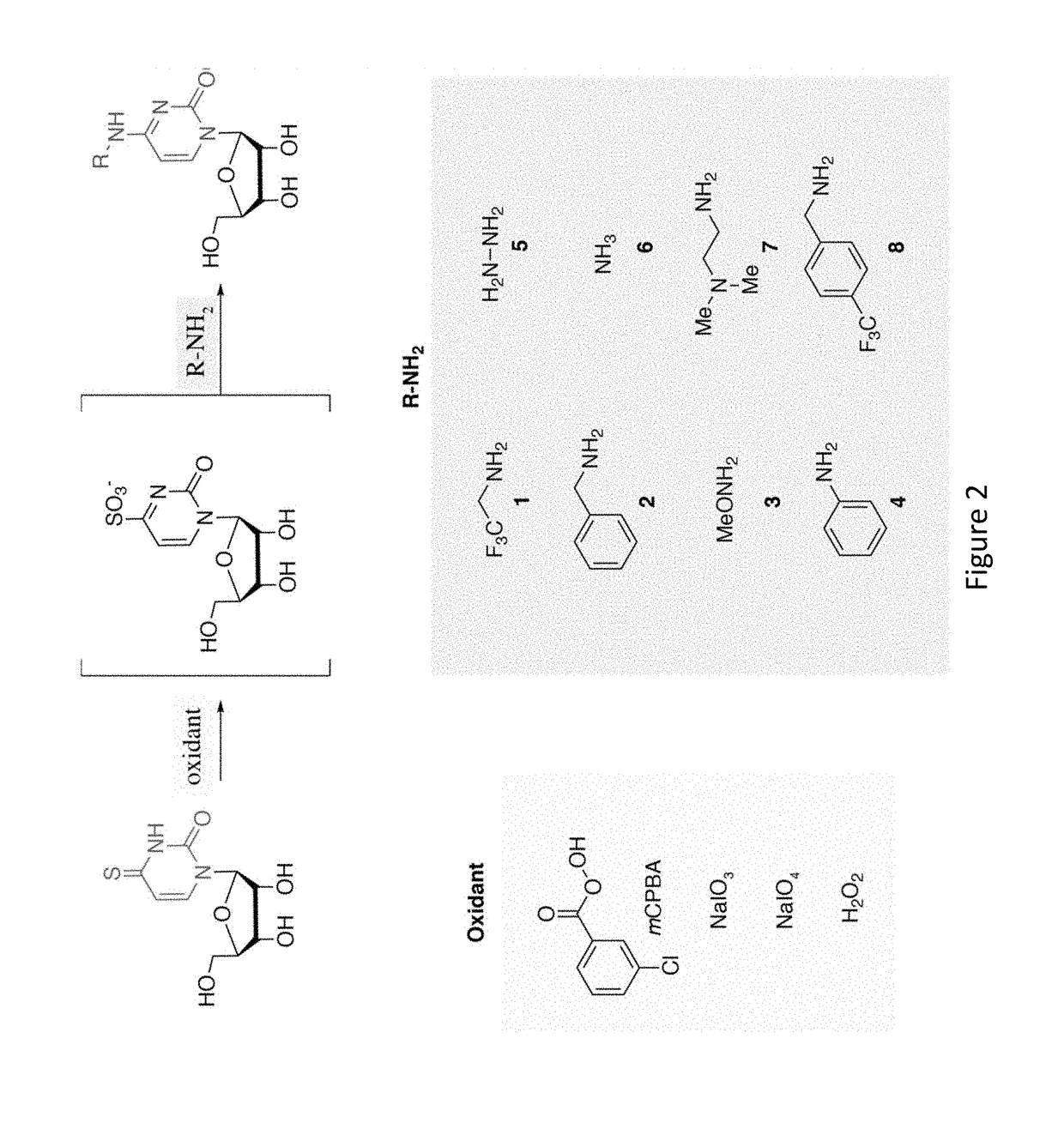
Time Lapse Sequencing: A convertible-nucleoside approach to enrichment-free analysis of RNA dynamics
ActiveUS20180282789A1Microbiological testing/measurementNucleophilic aromatic substitutionNucleoside
The invention provides methods and compositions for generating mutations in new nucleic acid molecules through incorporation of a transformable nucleoside into the nucleic acid and subsequent transformation of the nucleoside through oxidative-nucleophilic-aromatic-substitution chemistry, referred to as TimeLapse chemistry. The invention further provides methods for detecting the mutations, referred to as TimeLapse-seq.
Owner:YALE UNIV
Preparation method of intermediate for leukemia treatment medicines
InactiveCN109134366APlay a catalytic roleImprove catalytic performanceOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsFiltrationTrifluoroacetic acid
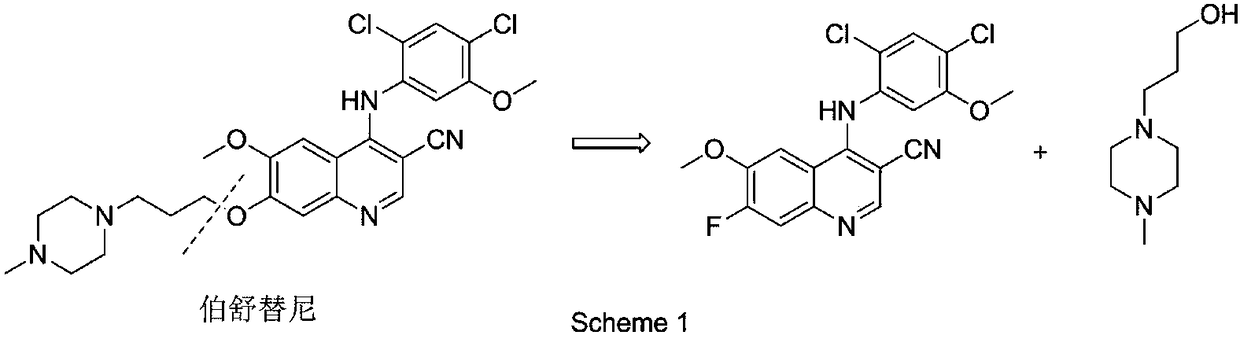
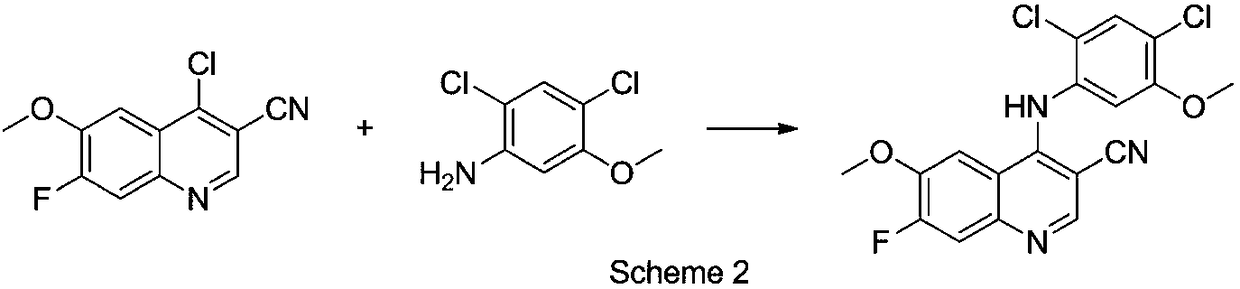
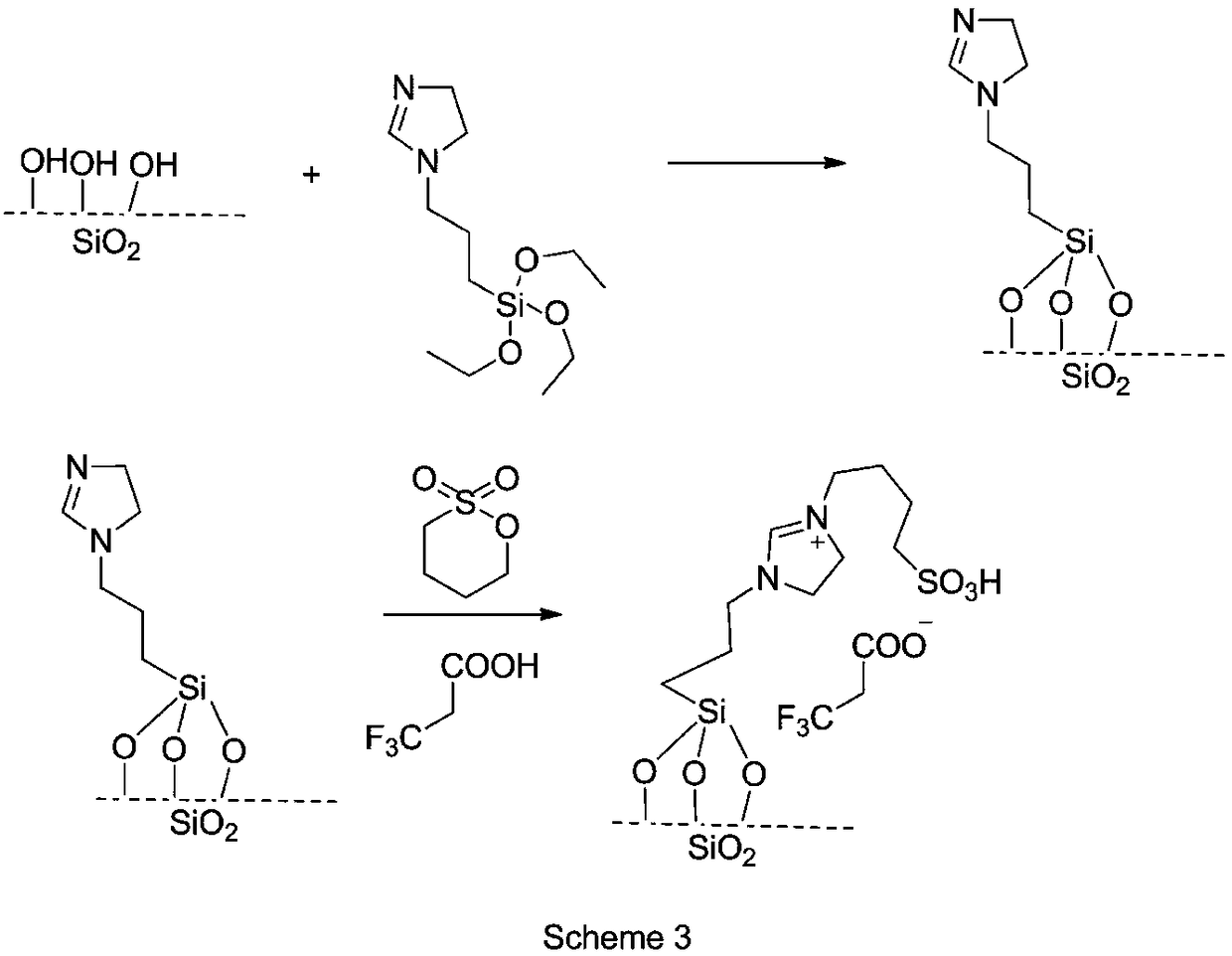
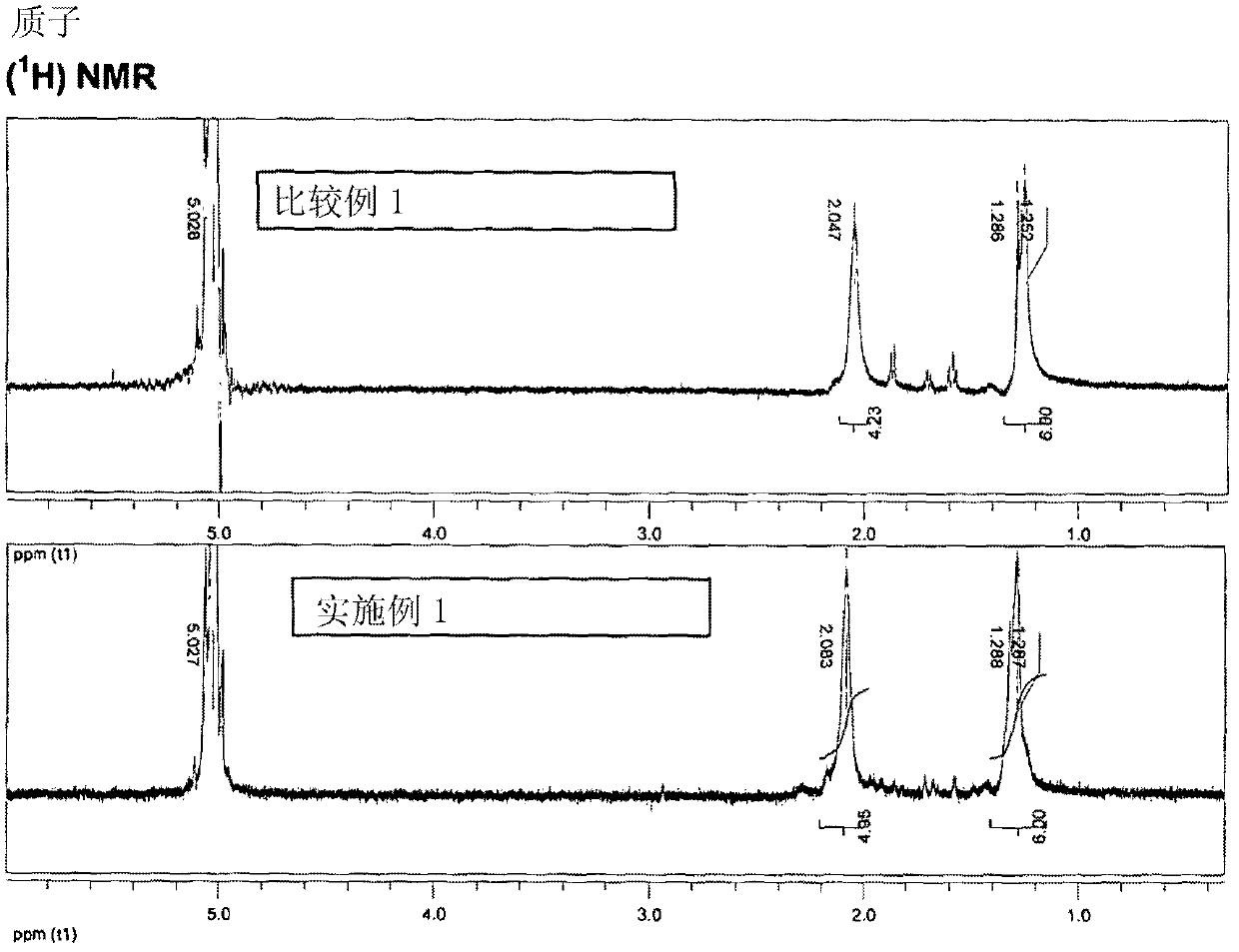
The invention belongs to the technical field of chemical medicines, and specifically relates to a preparation method of an intermediate for leukemia treatment medicines. The method comprises the stepsof supporting N-[3-(triethoxysilyl)propyl]-4,5-dihydroimidazole onto silica, and then reacting with 1,4-butyl sultone and trifluoroacetic acid to generate a silica gel supported acidic ionic liquid;and catalyzing the reaction of 4-chloro-6-methoxy 7-fluoro-quinoline-3-carbonitrile and 2,4-dichloro-5-methoxyaniline by using the acidic ionic liquid as an acidic catalyst to generate an intermediate 4-(2,4-dichloro-5-methoxyphenylamine)-7-fluoro-6-methoxyquinoline-3-nitrile for leukemia treatment medicines. The yield is up to 90.2%, and the process is environment-friendly; and the silica gel supported acidic ionic liquid can be removed from the reaction system by simple filtration, and can catalyze the aromatic nucleophilic substitution reaction of various substrates.
Owner:邹永旗
Synthesis method using ionic liquids
InactiveCN102471477BOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsNucleophilic aromatic substitution
The disclosure herein provides methods for performing nucleophilic aromatic substitution reactions in ionic liquids and forming polymeric materials.
Owner:INVISTA TECHNOLOG IES S A R L
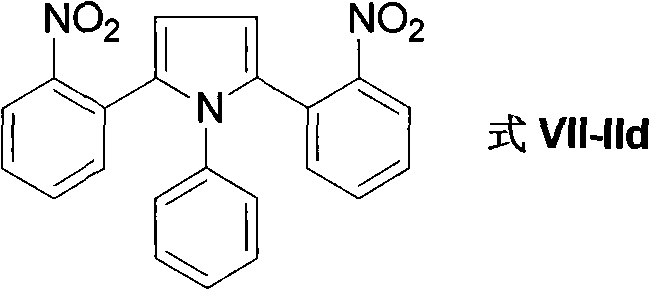
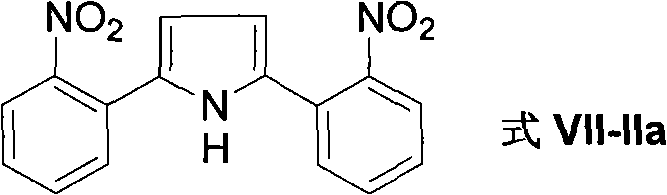
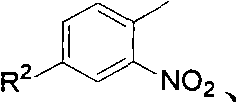
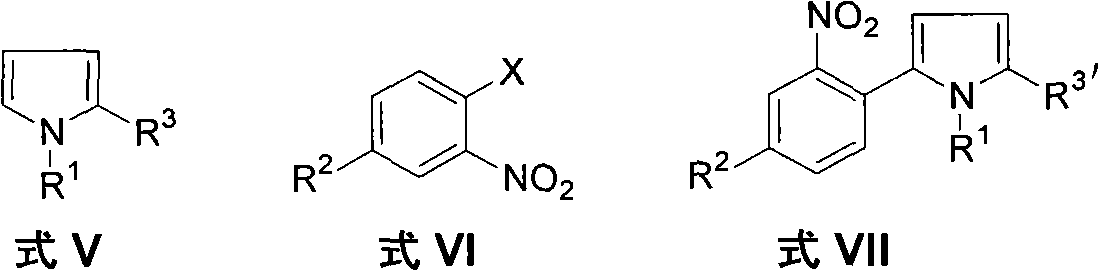
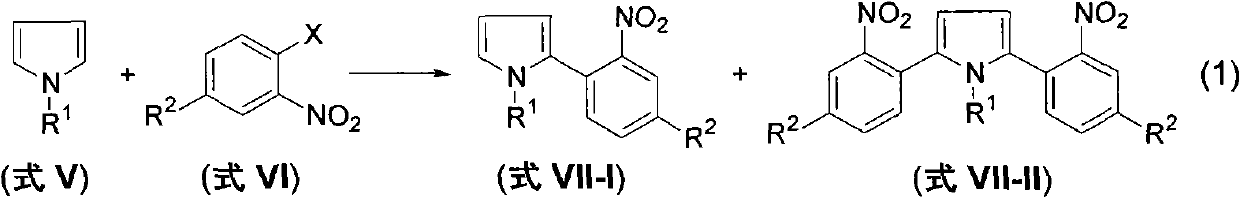
Method for preparing 2-(2-nitrophenyl) pyrrole and 2,5-bis(2-nitrophenyl) pyrrole compounds
The invention discloses a method for preparing 2-(2-nitrophenyl) pyrrole and 2,5-bis(2-nitrophenyl) pyrrole compounds. In the method, a compound shown in a general formula V and a compound shown in a general formula VI undergo reaction similar to nucleophilic aromatic substitution reaction under the alkali condition to obtain a compound shown in a general formula VII in a mode of high efficiency and high regioselectivity. The reaction has high regioselectivity; even if an N-H pyrrole compound or a pyrrole compound with an N-H substituent group is used as a substrate, the aromatic substitution reaction can still be performed at 2-position and 5-position of the pyrrole compound to obtain a C-arylation product in a mode of high regioselectivity; and no N-arylation product is generated. Thus, the characteristic ensures that the reaction can be used in the pyrrole compound which contains activated hydrogen, thereby expanding the application scope of the method.
Owner:胡跃飞
Method for preparing 2-(2-nitrophenyl) pyrrole and 2,5-bis(2-nitrophenyl) pyrrole compounds
The invention discloses a method for preparing 2-(2-nitrophenyl) pyrrole and 2,5-bis(2-nitrophenyl) pyrrole compounds. In the method, a compound shown in a general formula V and a compound shown in a general formula VI undergo reaction similar to nucleophilic aromatic substitution reaction under the alkali condition to obtain a compound shown in a general formula VII in a mode of high efficiency and high regioselectivity. The reaction has high regioselectivity; even if an N-H pyrrole compound or a pyrrole compound with an N-H substituent group is used as a substrate, the aromatic substitutionreaction can still be performed at 2-position and 5-position of the pyrrole compound to obtain a C-arylation product in a mode of high regioselectivity; and no N-arylation product is generated. Thus,the characteristic ensures that the reaction can be used in the pyrrole compound which contains activated hydrogen, thereby expanding the application scope of the method.
Owner:胡跃飞
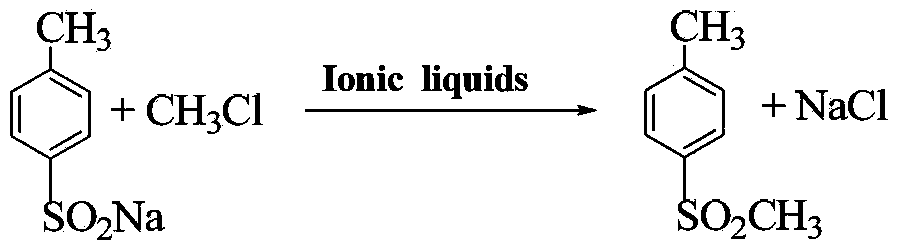
Method for preparing 4-methylsulfonyltoluene
ActiveCN102432509BIncrease the effective concentrationHigh purityOrganic chemistryOrganic compound preparationSodium bicarbonateSolvent
Owner:浙江嘉福新材料科技有限公司
Method for preparing chemical compounds of interest by nucleophilic aromatic substitution of aromatic carboxylic acid derivatives supporting at least one electro-attractive group
InactiveUS20120316337A1Optimize the numberNot producingOrganic compound preparationAmino-carboxyl compound preparationLeaving groupOrtho position
Method for preparing carboxylic acid derivatives by aromatic nucleophilic substitution, in which a carboxylic acid derivative having a single carboxyl functional group, or one of the salts thereof, the carboxylic acid derivative having, in the ortho position of the carboxyl functional group, a leaving group, which is preferably an atom of fluorine or of chlorine or an alkoxy group, chiral or not, preferably a methoxy group, the carboxylic acid derivative not being substituted by an electro attractive group other than the leaving group if any; is reacted with a reactant MNu, where M is a metal and Nu is a nucleophile, chiral or not, the aromatic nucleophilic substitution reaction being carried out without a catalyst and without a step of protecting / deprotecting the acid functional group of the starting compound.
Owner:UNIVERSITE DU MAINE
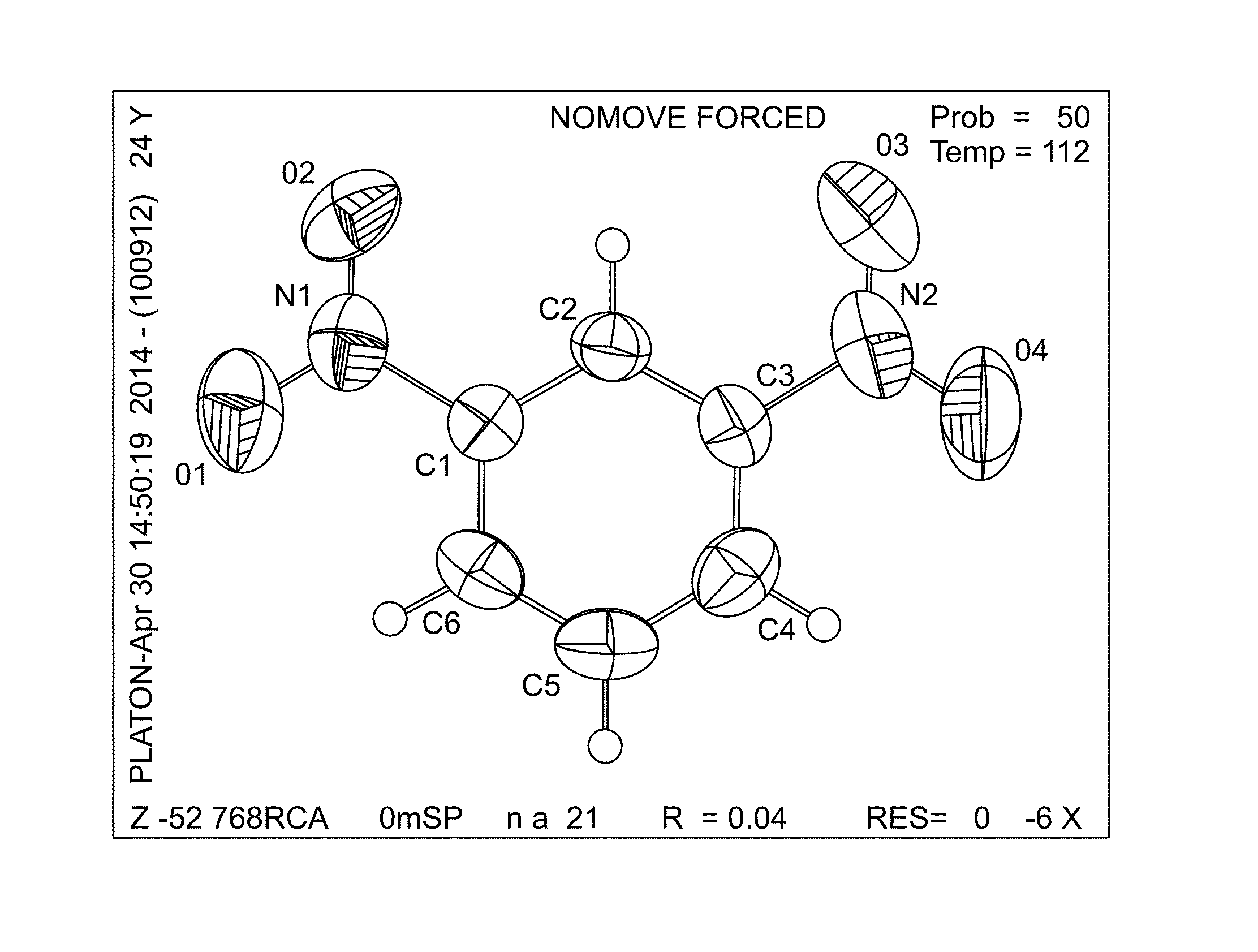
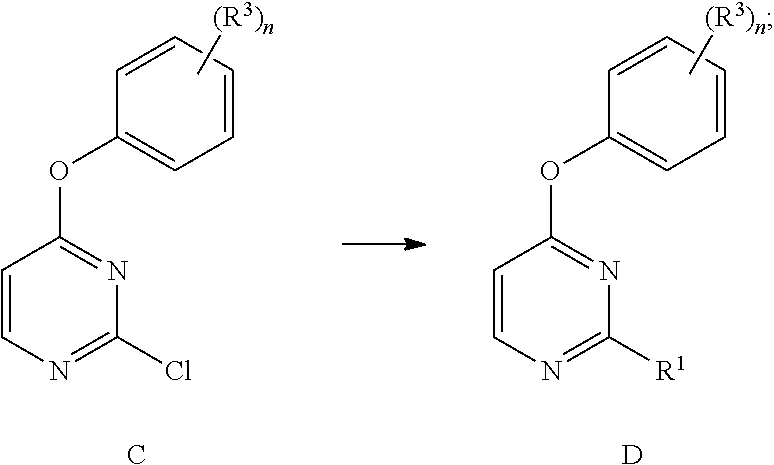
(1r,4r,7r)-7-amino-2-azabicyclo[2,2,1]heptane derivative and preparation method
ActiveCN113698403BAvoid inhibitionOrganic active ingredientsSenses disorderAutoimmune conditionAzabicyclane
Owner:NANJING GENTAI PHARMA TECH CO LTD
Preparation method of pyridine derivative
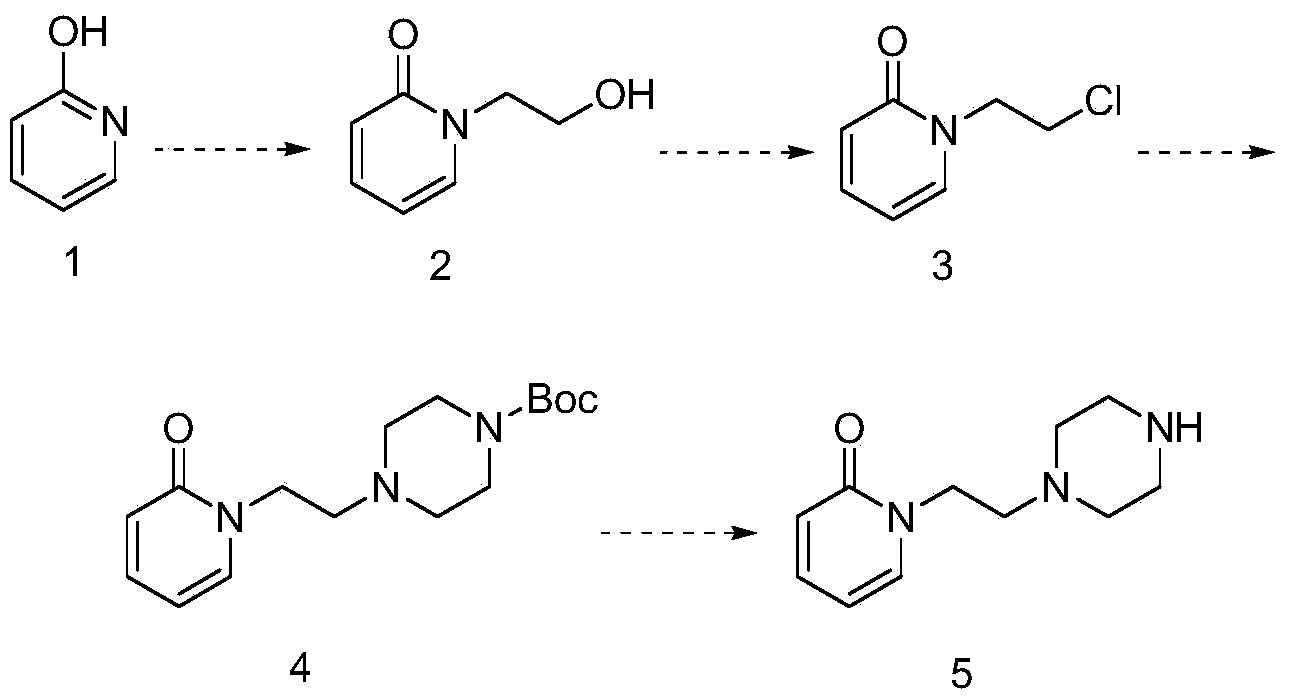
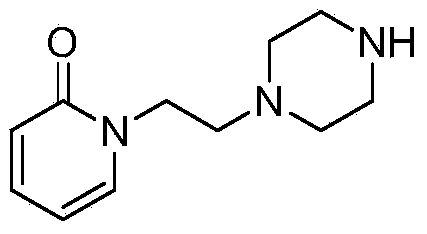
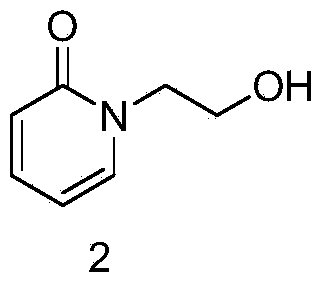
The invention discloses a preparation method of a pyridine derivative, and especially relates to a preparation method of 1-(2-(piperazine-1-group)ethyl)pyridine-2(1H)-ketone, the method takes 2-pyridone as an initial raw material, and 2-pyridone is subjected to nucleophilic substitution, chlorination, nucleophilic substitution and Boc removal to obtain the target product, and the compound is an important medicine intermediate.
Owner:湖南华腾制药有限公司
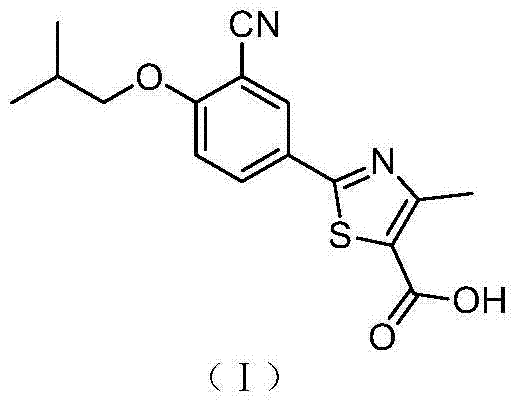
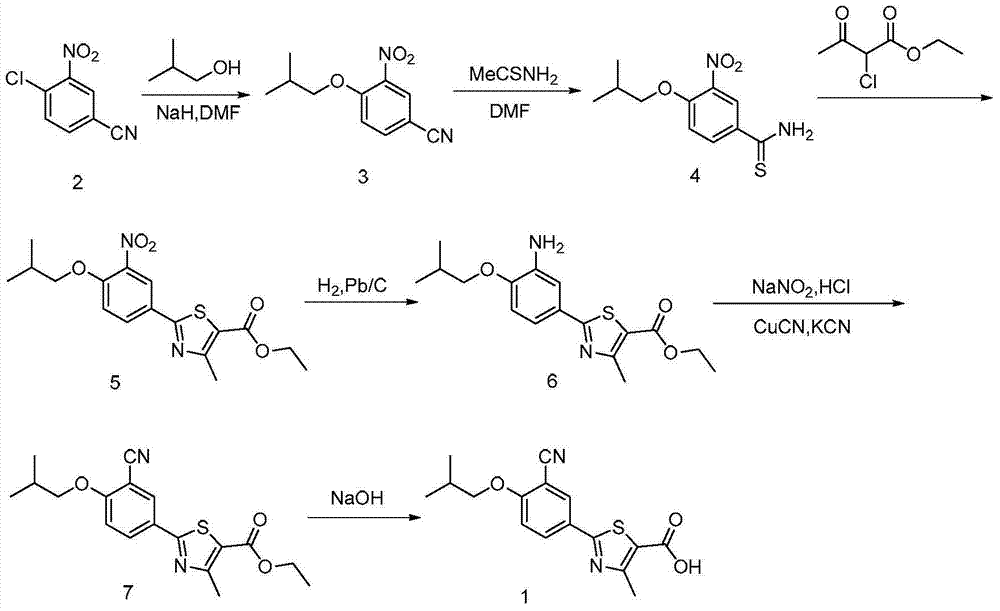
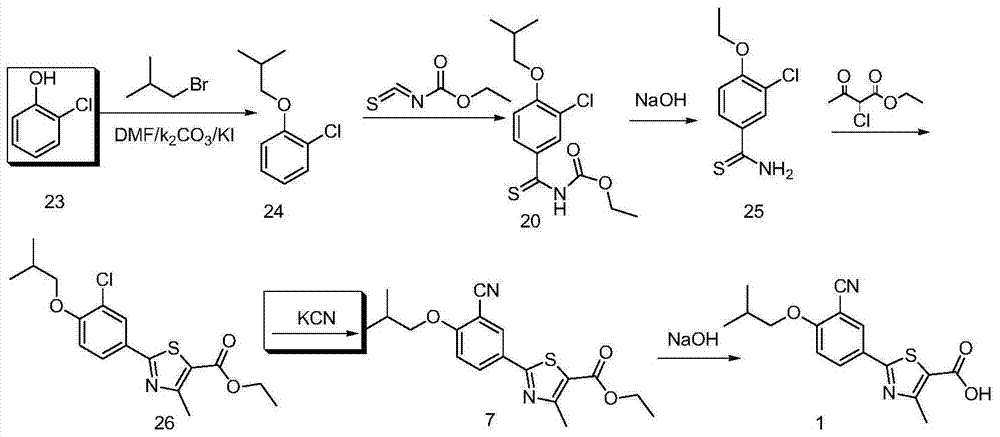
A kind of synthetic method of febuxostat
ActiveCN103910695BIncrease profitLow costOrganic chemistryHydrolysisNucleophilic aromatic substitution
The invention discloses a synthetic method of febuxostat. The synthetic method mainly comprises the following steps: (1) by taking a compound 4-hydroxythiobenzamide in a formula II as a raw material, preparing a compound in a formula III through cyclization reaction; (2) carrying out nucleophilic aromatic substitution reaction on the compound in the formula III to obtain a compound in a formula IV; (3) carrying out substitution reaction on the compound in the formula IV to obtain a compound in a formula V; (4) carrying out cyanation reaction on the compound in the formula V to obtain a compound in a formula VI; (5) carrying out hydrolysis reaction on the compound in the formula VI to obtain a compound febuxostat in a formula I. The preparation method disclosed by the invention has the advantages of being easy in process and suitable for industrial production, and the synthesized product febuxostat has the advantages of high purity and high yield.
Owner:CHONGQING KERUI PHARMA GRP
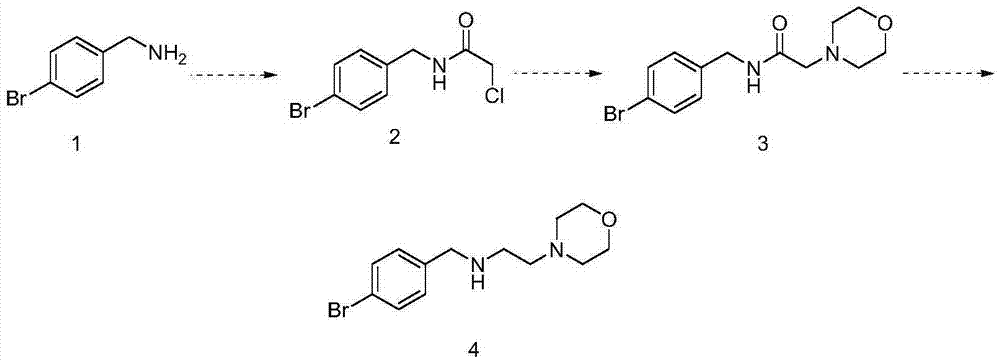
Preparation method for morpholine derivative
InactiveCN106854188ASynthetic method is fastEfficient synthesis methodOrganic chemistryMorpholineOrganic synthesis
The invention especially relates to a preparation method for a morpholine derivative, i.e., N-(4-bromobenzyl)-2-morpholineethylamine, belonging to the field of organic synthesis. According to the method, 4-bromobenzylamine is used as a starting raw material and subjected to acylation, nucleophilic substitution and reduction so as to prepare the morpholine derivative. The prepared morpholine derivative can enrich organic-synthesis molecule building blocks.
Owner:湖南华腾制药有限公司
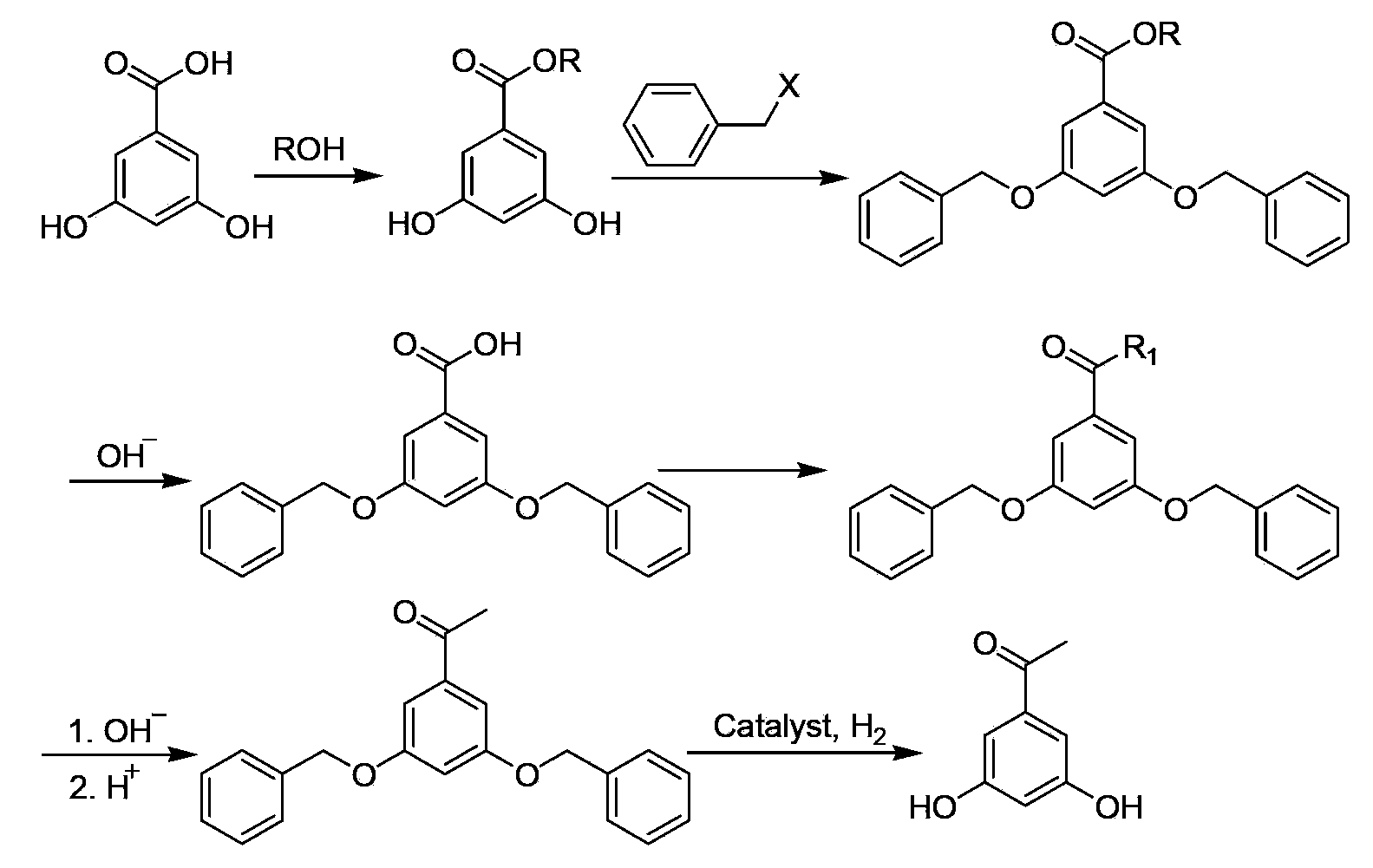
Preparation method of 3, 5-resacetophenone
InactiveCN102675075BSimple preparation processLow costOrganic compound preparationCarbonyl compound preparationBenzoic acidHydrolysis
The invention discloses a preparation method of 3,5-resacetophenone, which takes 3,5-dihydroxy-benzoic acid as a raw material, and the 3,5-resacetophenone can be prepared by esterification, benzylation, hydrolysis, nucleophilic substitution, hydrolysis, decarboxylation and debenzylation. The preparation method is mild in preparation condition, simple in technology, low in cost and higher in yield, and has higher industrial application value.
Owner:ZHEJIANG UNIV
(1R, 4R, 7R)-7-amino-2-azabicyclo [2, 2, 1] heptane derivative and preparation method thereof
ActiveCN113698403AAvoid inhibitionOrganic active ingredientsSenses disorderAutoimmune conditionAutoimmune disease
The invention belongs to the field of medicine, and discloses a (1R, 4R, 7R)-7-amino-2-azabicyclo [2, 2, 1] heptane derivative and a preparation method thereof. The preparation method comprises the following steps of: (S1) carrying out nucleophilic aromatic substitution reaction on compounds I-1 and I-2 to obtain a compound A-1; (S2) treating the compound A-1 with hydrochloric acid or trifluoroacetic acid for deprotection to obtain a compound A-2; and (S3) carrying out coupling reaction on the compound A-2 and organic acid or acyl chloride, or carrying out reaction on the compound A-2 and amine and triphosgene, or carrying out reaction on the compound A-2 and amine and carbonyl diimidazole to obtain the (1R, 4R, 7R)-7-amino-2-azabicyclo [2, 2, 1] heptane derivative. The (1R, 4R, 7R)-7-amino-2-azabicyclo [2, 2, 1] heptane derivative developed by the invention can avoid inhibition of JAK2 and selectively inhibit JAK1 or JAK1 / Tyk2, and has important significance in treatment of autoimmune diseases.
Owner:NANJING GENTAI PHARMA TECH CO LTD
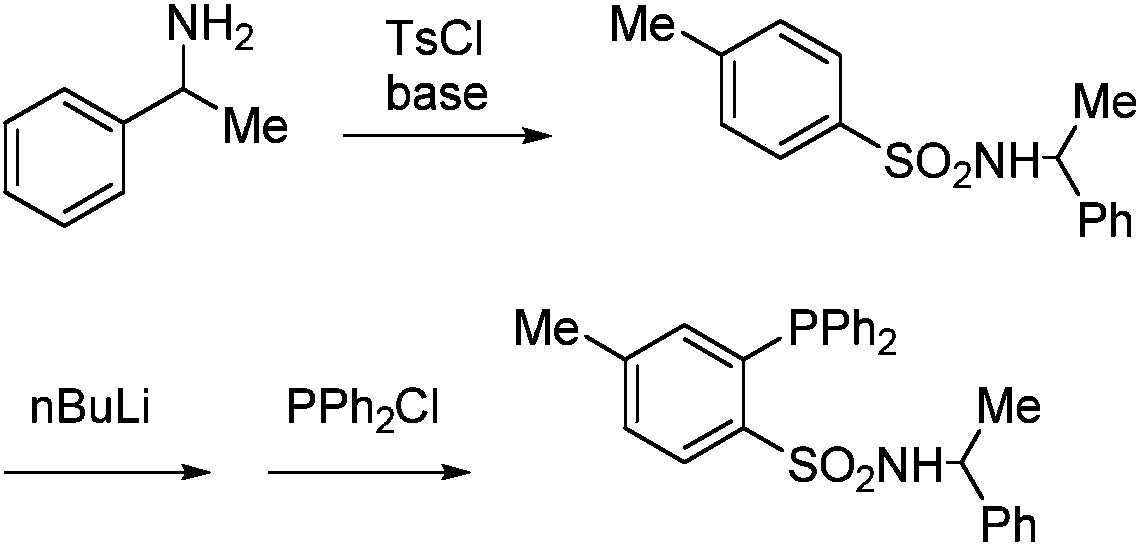
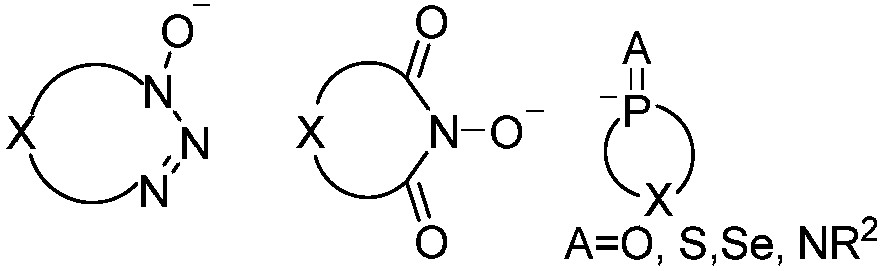
Method for producing optically active 2, 3-bisphosphinopyrazine derivative and method for producing optically active phosphine transition metal complex
ActiveUS20200087330A1High optical purityHigh yieldGroup 5/15 element organic compoundsPyrazineCarboxylic acid
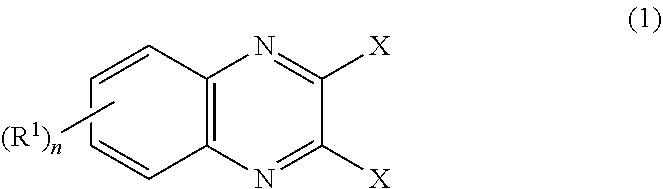
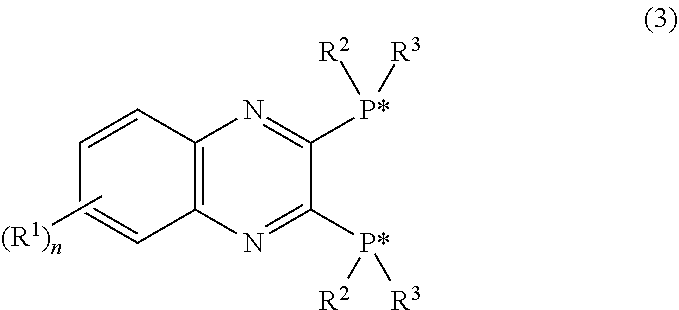
In the method for producing an optically active 2,3-bisphosphinopyrazine derivative of the present invention, an optically active 2,3-bisphosphinopyrazine derivative represented by the following formula (3) is produced by the step of: preparing solution A containing 2,3-dihalogenopyrazine represented by the following formula (1)and a carboxylic acid amide coordinating solvent, lithiating an optically active R- or S-isomer of a hydrogen-phosphine borane compound represented by the following formula (2)to give a lithiated phosphine borane compound; adding solution B containing the lithiated phosphine borane compound to the solution A to perform an aromatic nucleophilic substitution reaction; and then performing a deboranation reaction.(For symbols in the formulas, see the description.)
Owner:NIPPON CHECMICAL IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
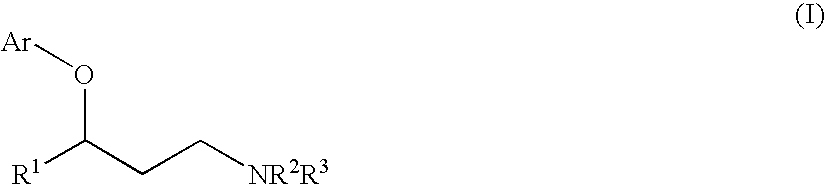
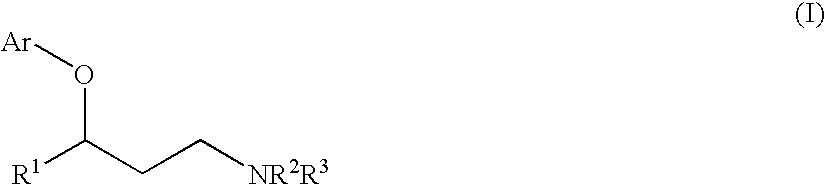

![(1r,4r,7r)-7-amino-2-azabicyclo[2,2,1]heptane derivative and preparation method (1r,4r,7r)-7-amino-2-azabicyclo[2,2,1]heptane derivative and preparation method](https://images-eureka.patsnap.com/patent_img/3e4eb44f-3fe3-453a-a094-1efc2fe4ff04/DEST_PATH_IMAGE004.png)
![(1r,4r,7r)-7-amino-2-azabicyclo[2,2,1]heptane derivative and preparation method (1r,4r,7r)-7-amino-2-azabicyclo[2,2,1]heptane derivative and preparation method](https://images-eureka.patsnap.com/patent_img/3e4eb44f-3fe3-453a-a094-1efc2fe4ff04/DEST_PATH_IMAGE006.png)
![(1r,4r,7r)-7-amino-2-azabicyclo[2,2,1]heptane derivative and preparation method (1r,4r,7r)-7-amino-2-azabicyclo[2,2,1]heptane derivative and preparation method](https://images-eureka.patsnap.com/patent_img/3e4eb44f-3fe3-453a-a094-1efc2fe4ff04/DEST_PATH_IMAGE008.png)
![(1R, 4R, 7R)-7-amino-2-azabicyclo [2, 2, 1] heptane derivative and preparation method thereof (1R, 4R, 7R)-7-amino-2-azabicyclo [2, 2, 1] heptane derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/84585d81-78b7-4265-9fda-31f4d11483fe/FDA0002501678860000011.png)
![(1R, 4R, 7R)-7-amino-2-azabicyclo [2, 2, 1] heptane derivative and preparation method thereof (1R, 4R, 7R)-7-amino-2-azabicyclo [2, 2, 1] heptane derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/84585d81-78b7-4265-9fda-31f4d11483fe/FDA0002501678860000012.png)
![(1R, 4R, 7R)-7-amino-2-azabicyclo [2, 2, 1] heptane derivative and preparation method thereof (1R, 4R, 7R)-7-amino-2-azabicyclo [2, 2, 1] heptane derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/84585d81-78b7-4265-9fda-31f4d11483fe/FDA0002501678860000031.png)