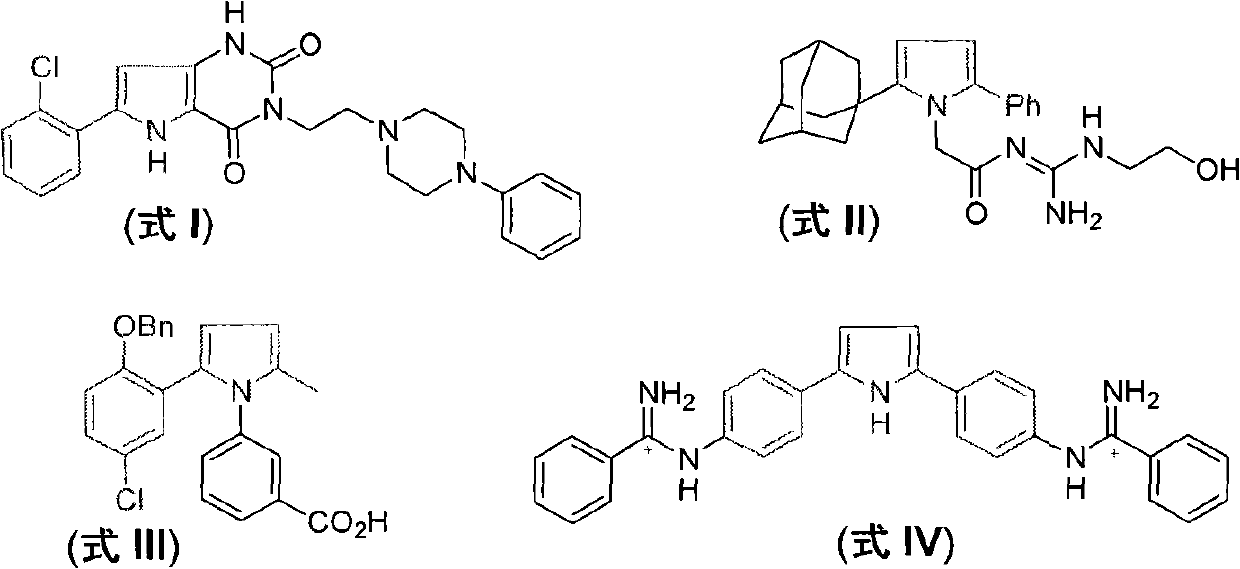
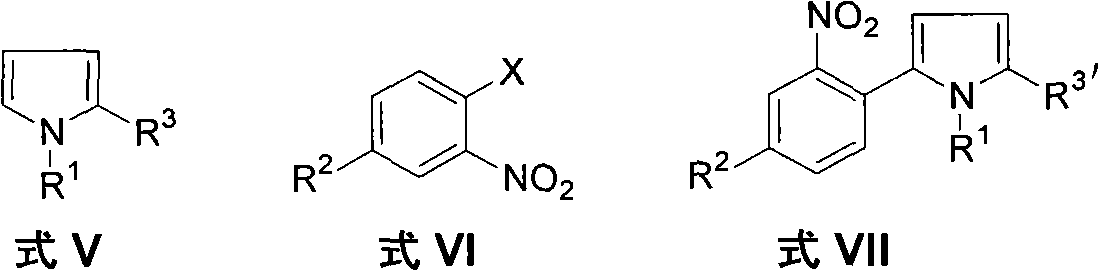
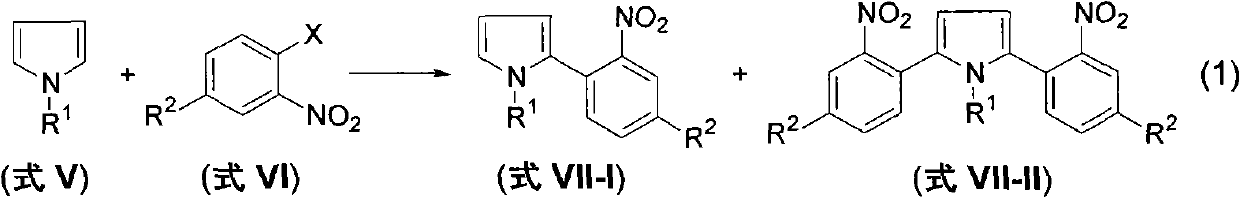
Method for preparing 2-(2-nitrophenyl) pyrrole and 2,5-bis(2-nitrophenyl) pyrrole compounds
A kind of technology of compound of general formula, alkyl carbonyl, applied in the field of preparing 2-pyrroles and 2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] The preparation of embodiment 1,2-(2-nitrophenyl) pyrrole (formula VII-Ia)
[0115]
[0116] Pyrrole (0.54g, 8.0mmol), 2-bromonitrobenzene (0.40g, 2.0mmol), Cs 2 CO 3 The mixed system of (1.30g, 4mmol) and acetonitrile (30mL) was refluxed and stirred under the protection of nitrogen. After the reaction was detected by TLC, the system was cooled to room temperature, the solid in the system was removed by filtration, and the filtrate was collected. The filtrate was eluted by flash column chromatography [silica gel, PE (petroleum ether) / DCM (dichloromethane)] after the solvent was evaporated under reduced pressure, and concentrated under reduced pressure to obtain a yellow solid 2-(2-nitrophenyl) Pyrrole 0.30 g (81% yield).
[0117] mp: 40-41°C
[0118] 13 C NMR: δ 147.8, 132.2, 130.4, 126.9, 126.7, 126.0, 124.1, 120.4, 110.5, 109.8.
[0119] Elemental Analysis: Calcd.for C 10 h 8 N 2 o 2 : C, 63.82; H, 4.28; N, 14.89. Found: C, 63.87; H, 4.23; N, 14.83.
[0...
Embodiment 2
[0121] The preparation of embodiment 2,2-(2-nitrophenyl) pyrrole (formula VII-Ia)
[0122]
[0123] Pyrrole (0.54g, 8.0mmol), 2-iodonitrobenzene (0.50g, 2.0mmol), Cs 2 CO 3 The mixed system of (1.30g, 4mmol) and acetonitrile (30mL) was refluxed and stirred under the protection of nitrogen. After the reaction was detected by TLC, the system was cooled to room temperature, the solid in the system was removed by filtration, and the filtrate was collected. The filtrate was eluted by flash column chromatography [silica gel, PE (petroleum ether) / DCM (dichloromethane)] after the solvent was evaporated under reduced pressure, and concentrated under reduced pressure to obtain a yellow solid 2-(2-nitrophenyl) Pyrrole 0.25 g (66% yield).
[0124] mp: 40-41°C
[0125] 13 C NMR: δ 147.8, 132.2, 130.4, 126.9, 126.7, 126.0, 124.1, 120.4, 110.5, 109.8.
[0126] Elemental Analysis: Calcd.for C 10 h 8 N 2 o 2 : C, 63.82; H, 4.28; N, 14.89. Found: C, 63.87; H, 4.23; N, 14.83.
[01...
Embodiment 3
[0128] The preparation of embodiment 3,2-(2-nitrophenyl)-N-methylpyrrole (formula VII-Ib)
[0129]
[0130] The preparation method is the same as in Example 1, N-methylpyrrole reacts with 2-bromonitrobenzene to obtain the product 2-(2-nitrophenyl)-N-methylpyrrole with a yield of 70%.
[0131] mp: 26-28°C (PE / EA).
[0132] 13 C N : δ149.7, 133.2, 132.2, 128.6, 128.1, 127.7, 123.9, 123.5, 109.4, 107.9, 34.1.
[0133] Elemental Analysis: Calcd.for C 11 h 10 N 2 o 2 : C, 65.34; H, 4.98; N, 13.85. Found: C, 65.60; H, 4.93; N, 13.81.
[0134] Indicates that the obtained compound is correct.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com