Preparation method for morpholine derivative
A technology of morpholine ethylamine and compounds, which is applied in the field of preparation of morpholine derivatives, that is, N--2-morpholine ethylamine, can solve problems such as synthesis difficulties and achieve the effect of an efficient synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
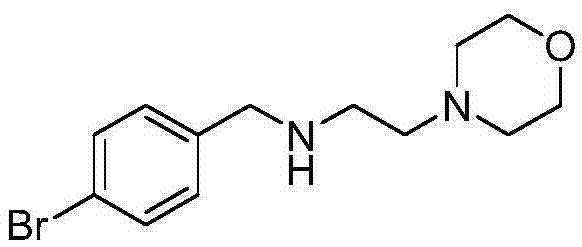
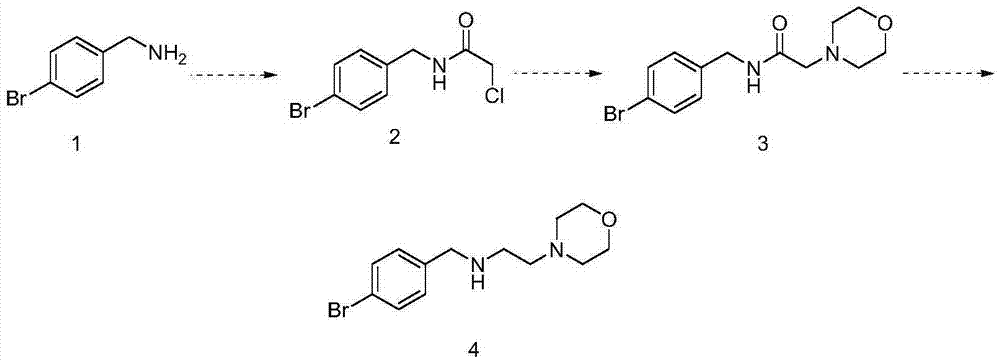
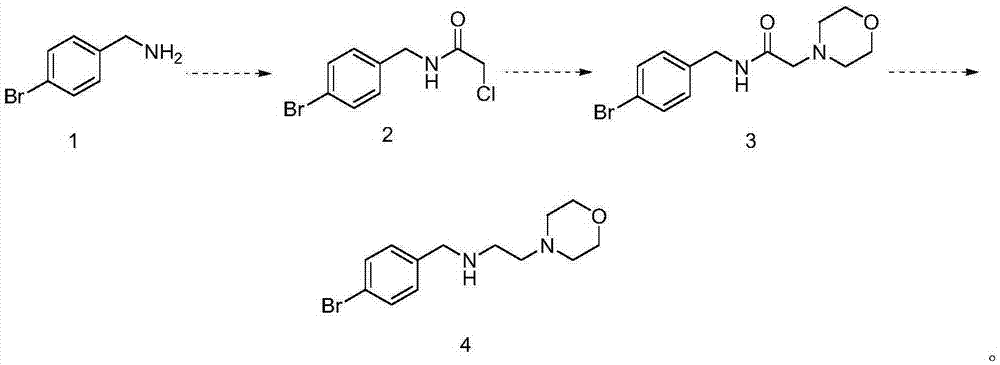
[0014] (1) Synthesis of (2-chloro-N-acetamido)-4-bromobenzylamine
[0015] 12g of 4-bromobenzylamine was added to 120ml of dichloromethane, cooled to 0°C, chloroacetyl chloride was added dropwise, stirred for 3 hours, water was added for extraction, liquid separation, drying, concentration, and the residue was separated on a column to obtain 14g ( 2-Chloro-N-acetamido)-4-bromobenzylamine.
[0016] (2) Synthesis of (2-morpholine-N-acetamido)-4-bromobenzylamine
[0017] 14g (2-chloro-N-acetamido)-4-bromobenzylamine was added to 110ml of N,N-dimethylformamide, 6g of potassium carbonate and 8g of morpholine were added, heated to reflux overnight, and cooled to Room temperature, concentrated, then added water and dichloromethane, extracted and separated, collected the organic phase, separated, dried and concentrated, and the residue was separated on a silica gel column to obtain 17g (2-morpholine-N-acetamido)-4- Bromobenzylamine.
[0018] (3) Synthesis of N-(4-bromobenzyl)-2-mor...
Embodiment 2
[0021] (1) Synthesis of (2-chloro-N-acetamido)-4-bromobenzylamine
[0022] 10g of 4-bromobenzylamine was added to 120ml of tetrahydrofuran, cooled to 5°C, chloroacetyl chloride was added dropwise, stirred for 4 hours, water was added for extraction, liquid separation, drying, concentration, and the residue was separated on a column to obtain 11g (2- Chloro-N-acetamido)-4-bromobenzylamine.
[0023] (2) Synthesis of (2-morpholine-N-acetamido)-4-bromobenzylamine
[0024] Add 11g (2-chloro-N-acetamido)-4-bromobenzylamine to 100ml toluene, add 5.7g triethylamine and 6.3g morpholine, heat to reflux overnight, cool to room temperature, concentrate, and Add water and ethyl acetate, extract and separate, collect the organic phase, separate, dry, concentrate, and separate the residue on a silica gel column to obtain 12.9 g of (2-morpholine-N-acetamido)-4-bromobenzylamine .
[0025] (3) Synthesis of N-(4-bromobenzyl)-2-morpholine ethylamine
[0026] Add 12.9g of (2-morpholine-N-aceta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com