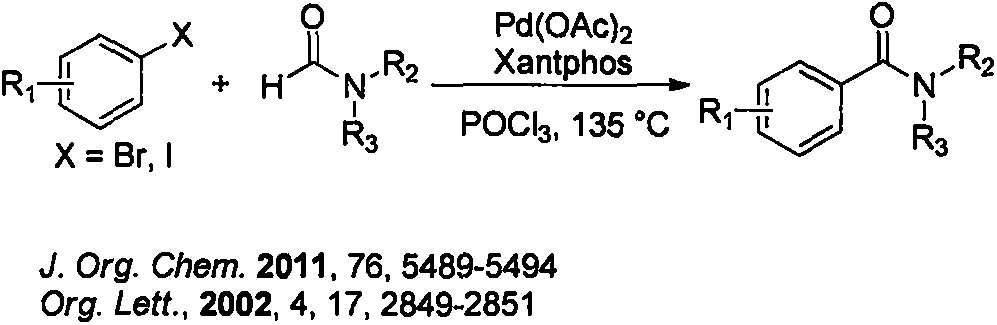
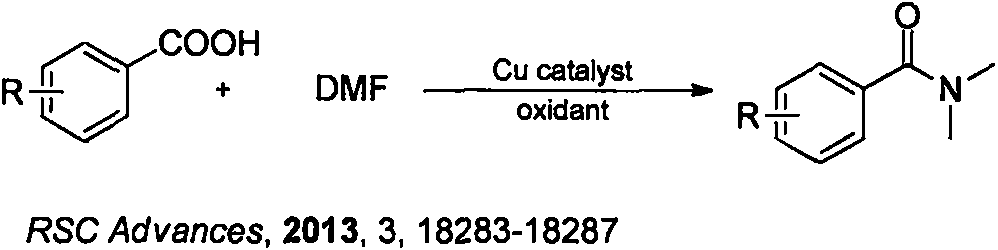
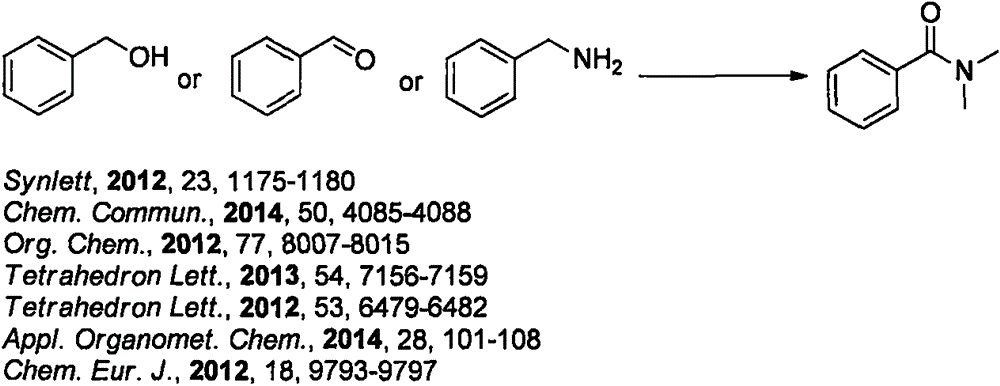
Method for synthesizing phosphorus-oxychloride-promoted amide compound
A technology of amide compounds and amide compounds, which is applied in the preparation of organic compounds, chemical instruments and methods, and the preparation of carboxylic acid amides, can solve the problems of high price, long reaction time, environmental pollution, etc. Yield, effect of widely applicable preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0031] Synthesis of N,N-dimethylbenzamide from benzoic acid and DMF:
[0032]
[0033] Add benzoic acid (0.036g, 0.3mmol), POCl 3 (lequiv), DMF (2mL), screw the bottle cap tightly, and react in an airtight manner at an external temperature of 120°C for 1 hour; monitor the completion of the reaction by gas chromatography; after the reaction liquid is cooled, add 10 mL of saturated Na 2 CO 3 The aqueous solution was extracted with ethyl acetate (3×10 mL), the organic phases were combined, dried over anhydrous magnesium sulfate, the solvent was removed by rotary evaporation, and a colorless and transparent Liquid, 90% yield.
[0034] 1 H NMR (300MHz, CDCl 3 )δ7.39(s, 5H), 3.10(s, 3H), 2.96(s, 3H); 13 C NMR (75MHz, CDCl 3 )δ171.76, 136.34, 129.61, 128.43, 127.11, 39.69, 35.43; MS (70eV, EI) m / z (EI) C 9 h 11 NO[M]: 149.19, 51(36), 77(100), 105(29), 148(56), 149(5).
example 2
[0036] Synthesis of N,N-dimethyl-3-methylbenzamide from 3-methylbenzoic acid and DMF:
[0037]
[0038] Add 3-methylbenzoic acid (0.041g, 0.3mmol), POCl3 (lequiv), DMF (2mL), screw the bottle cap tightly, and react in an airtight manner at an external temperature of 120°C for 1 hour; monitor the completion of the reaction by gas chromatography; after the reaction liquid is cooled, add 10 mL of saturated Na 2 CO 3 The aqueous solution was extracted with ethyl acetate (3×10 mL), the organic phases were combined, dried over anhydrous magnesium sulfate, the solvent was removed by rotary evaporation, and a colorless and transparent Liquid, 95% yield.
[0039] 1 H NMR (300MHz, CDCl 3 )δ7.23(dq, J=13.0, 7.4Hz, 4H), 3.04(d, J=39.5Hz, 6H), 2.37(s, 3H); 13 C NMR (75MHz, CDCl 3 )δ171.94, 138.28, 136.37, 130.28, 128.24, 127.73, 124.05, 39.68, 35.38, 21.45; MS (70eV, EI) m / z (EI) C 10 h 13 NO[M]: 163.22, 65(27), 91(100), 119(33), 162(28), 163(4).
example 3
[0041] Synthesis of N,N-dimethyl 4-hydroxybenzamide from 4-hydroxybenzoic acid and DMF:
[0042]
[0043] Add 4-hydroxybenzoic acid (0.041g, 0.3mmol), POCl 3 (lequiv), DMF (2mL), screw the bottle cap tightly, and react in an airtight manner at an external temperature of 120°C for 1 hour; monitor the completion of the reaction by gas chromatography; after the reaction liquid is cooled, add 10 mL of saturated Na 2 CO 3 The aqueous solution was extracted with ethyl acetate (3×10 mL), the organic phases were combined, dried over anhydrous magnesium sulfate, the solvent was removed by rotary evaporation, and a colorless and transparent Liquid, 55% yield.
[0044] 1 H NMR (300MHz, CDCl 3 )δ7.25-7.19 (m, 2H), 6.74-6.66 (m, 2H), 3.06 (d, J=19.0Hz, 6H); 13 C NMR (75MHz, CDCl 3 )δ172.82, 158.77, 129.20, 126.31, 115.50, 38.04 (d, J=321.6Hz); MS (70eV, EI) m / z (EI) C 9 h 11 NO 2 [M]: 165.19, 63(26), 65(100), 73(44), 93(83), 121(82), 164(28), 165(3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com