Preparation method of 3, 5-resacetophenone
A technology of dihydroxyacetophenone and dihydroxybenzoic acid, which is applied in the field of preparation of 3,5-dihydroxyacetophenone, can solve the problems of harsh conditions, difficult industrial application, low yield and the like, and achieves low cost and high production efficiency. The effect of simple process and strong industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
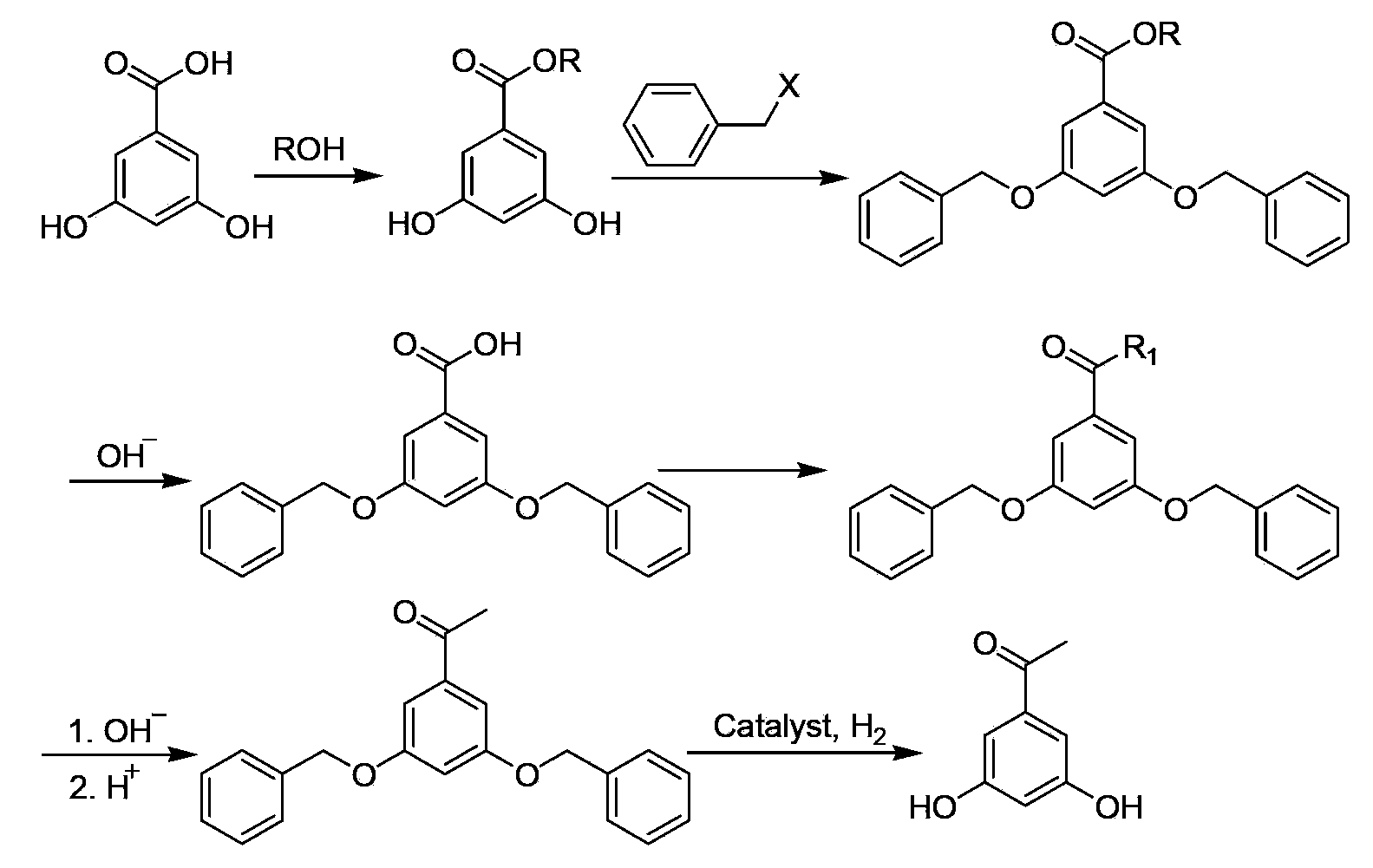
[0024] 1) Synthesis of ethyl 3,5-dihydroxybenzoate
[0025] Add 30.80g (200mmol) of 3,5-dihydroxybenzoic acid and 140mL of absolute ethanol to a 500mL four-neck flask in sequence, stir mechanically at room temperature until completely dissolved, slowly add 3.6mL of concentrated sulfuric acid dropwise, and heat to reflux for 8h. Ethanol was distilled off, after cooling to room temperature, 150 mL of methyl tert-butyl ether was added, followed by extraction with saturated aqueous solution of sodium bicarbonate (100 mL×2), saturated aqueous solution of sodium chloride (100 mL×1), and water (100 mL×2). The layer was dried over anhydrous magnesium sulfate overnight. Suction filtration, rotary evaporation of the filtrate, and vacuum drying gave 35.96 g of a white solid, yield 98.8%.
[0026] 2) Synthesis of ethyl 3,5-dibenzyloxybenzoate
[0027] Add 27.30g (150mmol) ethyl 3,5-dihydroxybenzoate, 60.00g (600mmol) potassium bicarbonate, 0.91g sodium iodide and 200mL acetone to a 500m...
Embodiment 2
[0037] 1) Synthesis of ethyl 3,5-dihydroxybenzoate
[0038] Add 30.80g (200mmol) of 3,5-dihydroxybenzoic acid and 170mL of absolute ethanol to a 500mL four-neck flask in sequence, stir mechanically at room temperature until completely dissolved, slowly add 2.4mL of concentrated sulfuric acid dropwise, and heat to reflux for 10h. Ethanol was distilled off, after cooling to room temperature, 150 mL of methyl tert-butyl ether was added, followed by extraction with saturated aqueous solution of sodium bicarbonate (100 mL×2), saturated aqueous solution of sodium chloride (100 mL×1), and water (100 mL×2). The layer was dried over anhydrous magnesium sulfate overnight. Suction filtration, rotary evaporation of the filtrate, and vacuum drying gave 35.33 g of white solid, yield 97.1%.
[0039] 2) Synthesis of ethyl 3,5-dibenzyloxybenzoate
[0040] Add 27.30g (150mmol) ethyl 3,5-dihydroxybenzoate, 72.45g (525mmol) potassium carbonate, 1.00g sodium iodide and 140mL acetone into a 500mL...
Embodiment 3
[0050] 1) Synthesis of ethyl 3,5-dihydroxybenzoate
[0051] Add 30.80g (200mmol) of 3,5-dihydroxybenzoic acid and 200mL of absolute ethanol to a 500mL four-neck flask in sequence, stir mechanically at room temperature until completely dissolved, slowly add 3.5mL of concentrated sulfuric acid dropwise, and heat to reflux for 12h. Ethanol was distilled off, after cooling to room temperature, 150 mL of methyl tert-butyl ether was added, followed by extraction with saturated aqueous solution of sodium bicarbonate (100 mL×2), saturated aqueous solution of sodium chloride (100 mL×1), and water (100 mL×2). The layer was dried over anhydrous magnesium sulfate overnight. Suction filtration, rotary evaporation of the filtrate, and vacuum drying gave 35.56 g of white solid, yield 97.7%.
[0052] 2) Synthesis of ethyl 3,5-dibenzyloxybenzoate
[0053] Add 32.55g (179mmol) ethyl 3,5-dihydroxybenzoate, 61.7g (447mmol) potassium carbonate, 1.50g sodium iodide and 165mL acetone to a 500mL fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com