Preparation method of 17hydroxy-pregnane-4-alkene-3,20-diketone-21-acetic ester
A technology of acetate and pregnan, which is applied in the field of preparation of 17-hydroxy-pregn-4-ene-3,20-dione-21-acetate, can solve the problems of low yield and high cost, and achieves a high yield High, stable yield and wide-ranging effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
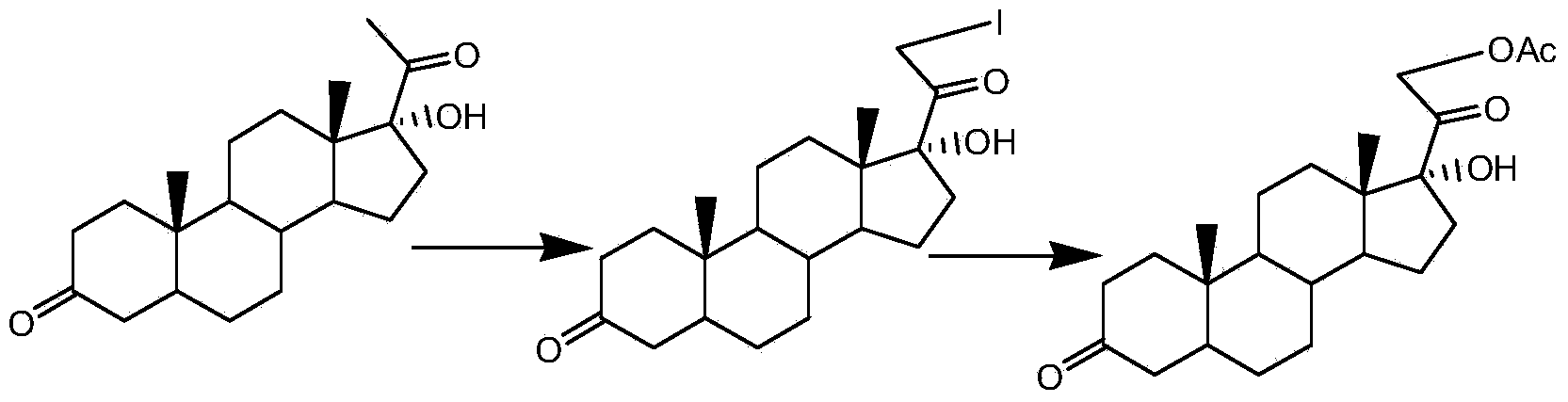
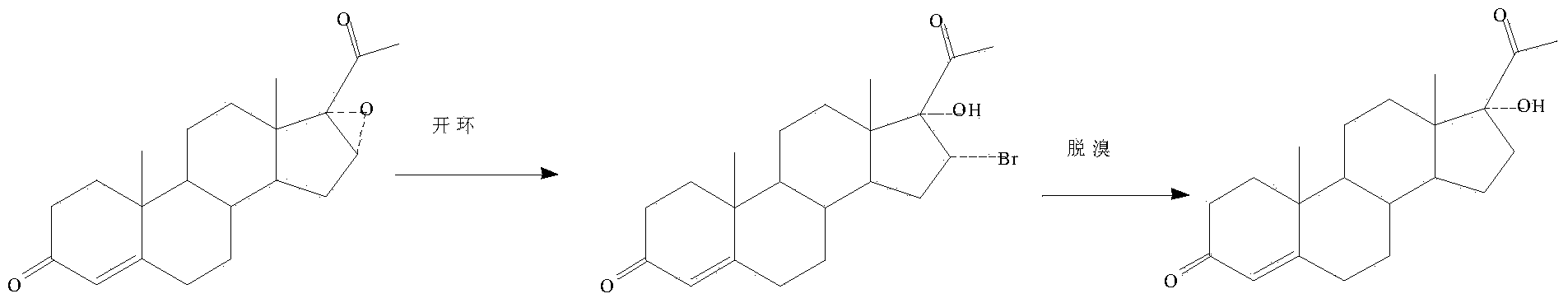
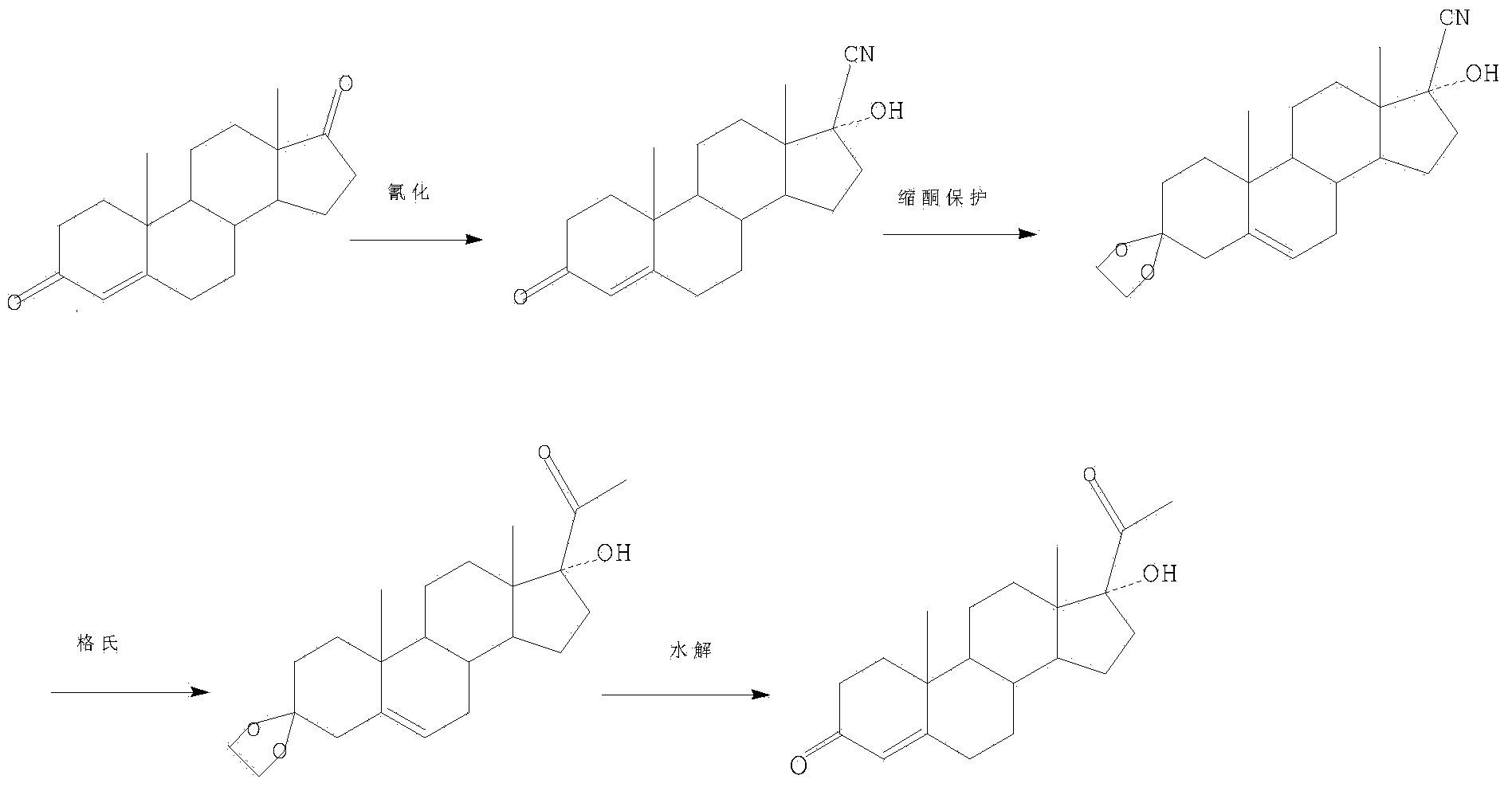
[0033] The reaction scheme of the present invention is as follows:
[0034]
[0035] Cyanation reaction
[0036] Put 100 milliliters of methanol, 50 grams of acetone cyanohydrin, and 100 grams of the compound represented by formula (II) into a three-necked flask. Turn on the stirring and slowly raise the temperature to 35°C. The prepared potassium carbonate solution was slowly added dropwise, and the temperature was controlled at 32° C. for 20 h. TLC detection confirmed that the raw material point is very small and the raw material point is no longer reduced, then cooled to 5°C, slowly passed the material into a beaker of 2 liters of water that had been cooled to below 10°C in advance, and stood still for more than 2h. Filter and wash with water until neutral. Add the filter cake to the prepared dilute hydrochloric acid, stir for 2 hours, filter, wash the filter cake with a large amount of water until PH = 6.0-6.5, filter, and discharge. The filter cake was transferred ...
Embodiment 2
[0044] Cyanation reaction
[0045] Put 100 milliliters of ethanol, 100 grams of sodium cyanide, and 100 grams of the compound represented by formula (II) into a three-necked flask. Turn on the stirring and slowly raise the temperature to 38°C. The prepared sodium hydroxide solution was slowly added dropwise, and the temperature was controlled at 38° C. for 20 h. TLC detection confirmed that the raw material point is very small and the raw material point is no longer reduced, then cooled to 0 °C, slowly passed the material into a beaker of 2 liters of water that had been cooled to below 10 °C in advance, and stood still for more than 2 hours. Filter and wash with water until neutral. Add the filter cake to the prepared dilute hydrochloric acid, stir for 2 hours, filter, wash the filter cake with a large amount of water until PH = 6.5, filter, and discharge. The filter cake was transferred to an oven and dried at 72°C for 12h. In crude cyanide. The yield is about 97%, HPLC≥...
Embodiment 3
[0053] Cyanation reaction
[0054] Put 100 milliliters of acetone, 70 grams of potassium cyanide, and 100 grams of the compound represented by formula (II) into a three-necked flask. Turn on the stirring and slowly raise the temperature to 50°C. The prepared sodium carbonate solution was slowly added dropwise, and the temperature was controlled at 50°C for 18 hours. TLC detection confirmed that the raw material point is very small and the raw material point is no longer reduced, then cooled to 0 °C, slowly passed the material into a beaker of 2 liters of water that had been cooled to below 10 °C in advance, and stood still for more than 2 hours. Filter and wash with water until neutral. Add the filter cake to the prepared dilute hydrochloric acid, stir for 2 hours, filter, wash the filter cake with a large amount of water until PH = 6.5, filter, and discharge. The filter cake was transferred to an oven and dried at 70°C for 12h. In crude cyanide. The yield is about 96%, H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com